Asian Textile Studies

Contents
Morinda
Morinda Species
Morinda Citrifolia L.
The Biogeography of Morinda
Morinda in India
The History of Morinda in Indonesia
Morinda in the Lesser Sunda Islands
The Cultivation and Harvesting of Morinda
Symplocos and other Natural Aluminium-Rich Mordants
Sources of Vegetable Oil
The Natural Morinda Dyeing Process
Morinda Dyeing in Indonesia
Morinda Dyeing in the Lesser Sunda Islands
Overdyeing with Morinda
Morindin and Morindone
The Anthroquinone Dyes
The Chemistry of Morinda Dyeing
Morinda Brown or Morinda Red?
Photo-Oxidation
Turkey Red
The History of Turkey Red
The Chemistry of Turkey Red Dyeing
Bibliography
Morinda
Red and brown dyes can be extracted from many different plants that grow in Malaysia and Indonesia, such as sappan (Caesalpinia sappan), yellow mangrove or tingi (Ceriops tagal), yellow flame tree or soga (Peltophorum pterocarpum), annatto (Bixa orellana), the engkerebai tree (Stylocoryne spp.), tamarind or asam, tenor, and various other tannin-rich tree barks. However the most important reddish-brown is that obtained from the outer bark of the roots of the small evergreen tree, Morinda citrifolia, sometimes called Indian mulberry or Noni. It is a native of Malaysia, Indonesia, and Queensland. In Indonesian, the dye is mainly called mangkudu, bangkudu, mengkudu or bengkudu.
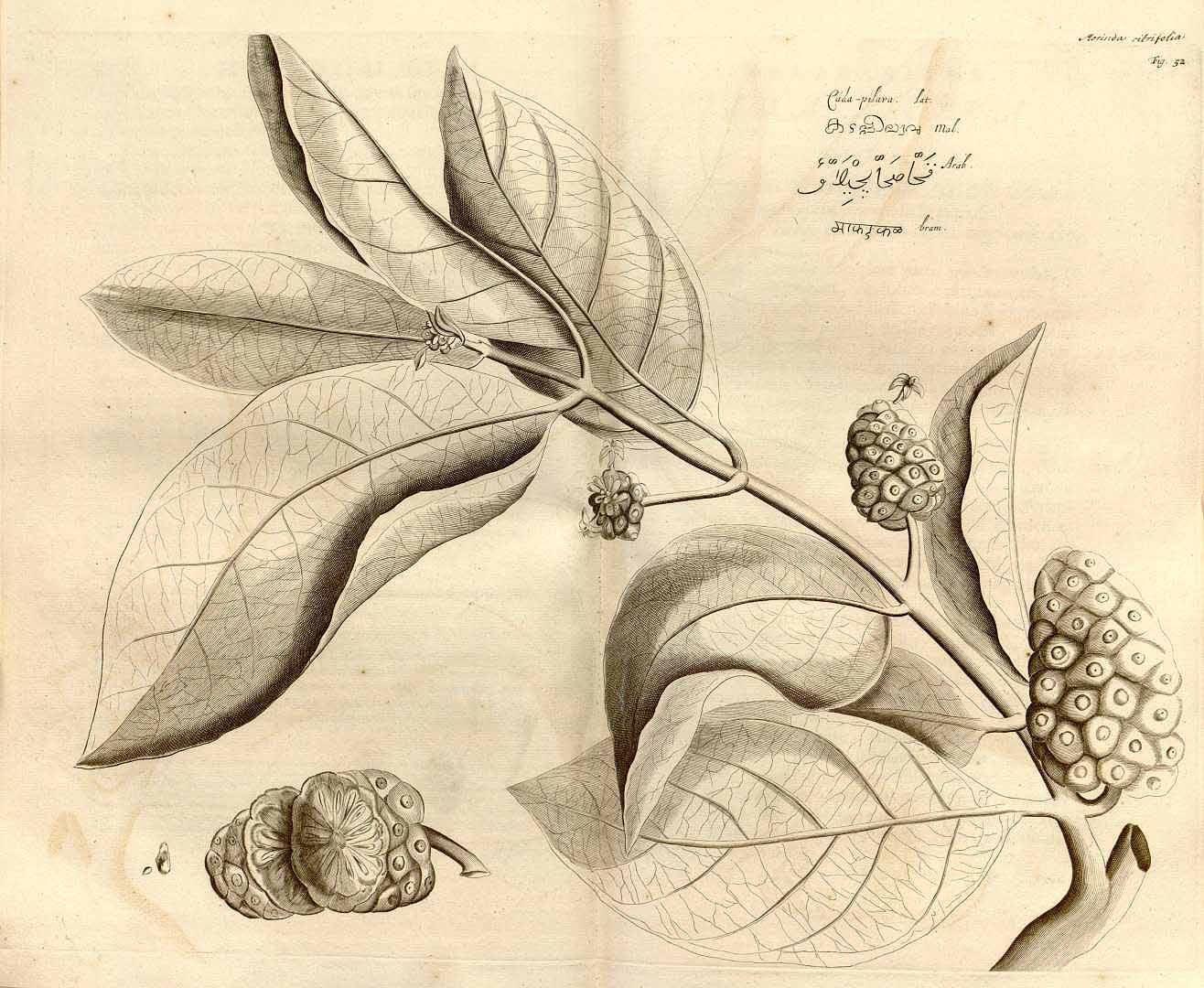
Morinda citrifolia
(H.A. van Rheede tot Drakestein, 1678. Hortus Indicus Malabaricus, vol. 1, t. 52)
The dyeing process is complicated, and far from being fully understood. It involves a two-phase mordanting process - the first requiring a vegetable oil and alkali, the second a source of aluminium and more alkali. The primary colorant is morindone, a member of the important anthraquinone family of dyestuffs. Even with a mordant, this binds weakly to cotton because cellulose is so inert. Consequently multiple stages of pre-mordanting, dyeing and drying are required to build up a deep colour.
Morinda Species
The Morinda genus, which was described by Linnaeus in 1753, is one of six controversial genera assigned to the pan-tropical tribe Morindeae. The latter contains about 160 species, all of which bear multiple fruits. Morindeae in turn belongs to the huge Rubiaceae family, most of which consists of flowering shrubs or small trees and infrequently herbs (Hutchinson 1973). Rubiaceous plants are distributed globally but are mainly tropical – they include coffee and numerous other dye plants such as madder, crosswort, gambier and bedstraw. Rubiaceous plants have a tendency to accumulate substantial amounts of anthraquinones, especially in their roots (Han et al 2001). In addition to providing a range of natural dyes they have also been found to have many useful medicinal properties.
The Morinda genus is composed of around 80 species, which are distributed throughout the tropics (Smith 1999). The name is derived from the Latin morus and indicus, meaning mulberry and India. Morinda is clearly the largest genus within the Morindeae tribe.
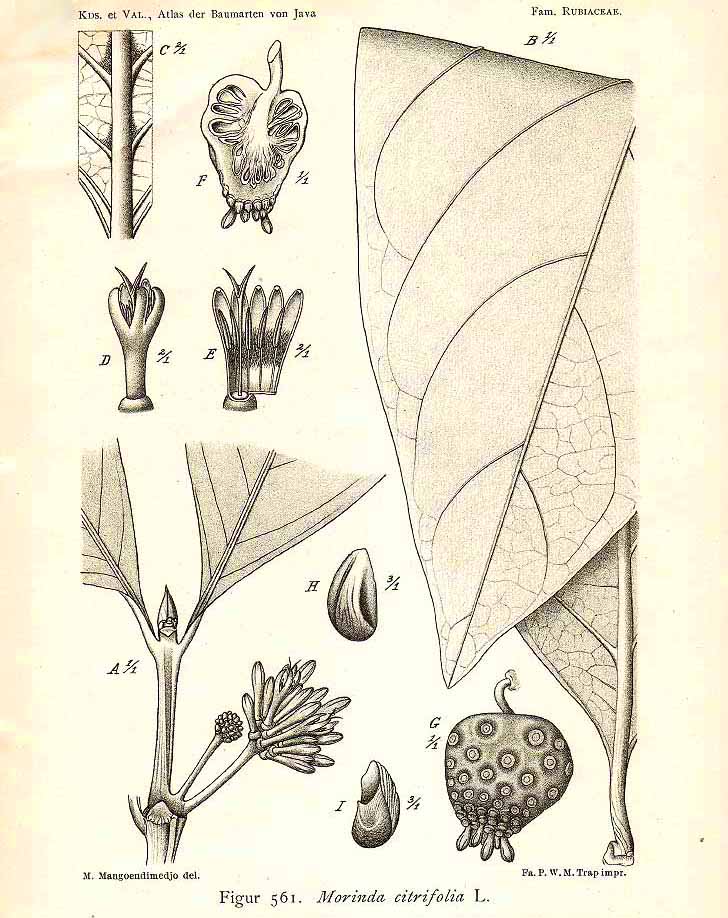
M. citrifolia L.
(Koorders and Valeton 1915. Atlas der Baumarten von Java, vol. 3, fig. 561)
The plants are all small trees or shrubs with leaves that have stalks (petiolate) and which range from papery to leathery. They produce stalked heads of flowers in which the carpels or ovaries (female reproductive organs) are fused together (i.e. they are syncarpus). The fruits are likewise fused together into an ellipsoidal structure known as a syncarp.
As with other Rubiaceae, many Morinda species have important pharmacological properties and their root bark contains valuable red and yellow dyes.
In India there are approximately eight endemic species of Morinda including M. citrifolia, M. tinctoria, M. angustifolia and M. umbellata, all of which seem to have been described by local names such as aal, a’l, ach or aich (Perkin and Everest 1918, 44). M. tomentosa was also considered important (Arya, Narayanan et al 2014). In many parts of India, especially Bengal, it was commercially cultivated for the production of red dye, marketed under the trade name Suranji (Perkin and Everest 1918, 44). However cultivation was severely curtailed by the arrival of aniline dyes in the early 1900s (Imperial Gazetteer of India vol. xxiv 1908, 179). M. tomentosa also occurs in Sri Lanka, China, Burma, Thailand and Java. Some authors refer to M. tomentosa as Thai morinda. It was once cultivated around villages in Northwest Thailand (Hanelt 2001, 1787). However some botanists believe that M. tomentosa is simply a synonym of M. tinctoria (Arya, Verma and Gupta 2015).
The climbing shrub M. umbellata, which has long pointed leaves, is widespread. It grows from India and Sri Lanka to China and Japan, as well as Southeast Asia, Indonesia and northern Australia. Its roots can be used for dyeing. In Malaysia it is called mengkudu akar or mengkudu hutan (morinda root or forest morinda), while M. citrifolia is referred to as mengkudu besar (Quattrocchi 1999, 1730).
The narrow leafed species M. elliptica, which grows in India, Malaysia, Southeast Asia and Burma, is also used to extract dye. In Malaysia it is called mengkudu kecil.
M. angustifolia is common in India, Nepal, Bangladesh and Burma. In the eastern Himalayas it is found up to an altitude of 1800m (Bhuyan and Saikia 2002). Depending on the metallic mordant used, its roots produce a range of dyes from yellow to brown and pink (Bhuyan, Saikia and Saikia 2001). A red pigment extracted from the roots of M. angustifolia has been found to contain morindone (Aobchey, Sriyam, et al 2002).
In Africa there are five endemic species of Morinda, all of which can be used for dyeing. The root bark of Morinda lucida is used to prepare a red dye in Nigeria and Gabon, especially by the Ashanti (Jansen 2005, 110). Some 18 anthraquinones have been isolated from its wood and bark, including alizarin, rubiadin, lucidin and morindin, along with two yellow anthraquinols. Morinda germinata is used in Côte d’Ivoire to dye cotton bright orange-red, while the root bark of M. longiflora and M. morindoides contain red anthraquinone colorants.
Morinda Citrifolia L.
Morinda citrifolia is commonly known as Indian mulberry, but is also called great morinda, noni, cheese fruit, hog apple and mouse’s pineapple. It is the only widely distributed member of the Morinda genus, found ranging from coastal Africa to India, Southeast Asia, Indonesia, Queensland, Polynesia and Hawai’i. It has been introduced into the Caribbean and Florida (Nellis 1994, 142).
It is a shrub or small tree that reaches maturity in around 20 to 25 years and grows to a height of from 3m to 10m. Growth is rapid in the first few years and then slows. The plants have dark glossy evergreen leaves, white flowers and a hard, knobbly, green fruit. The fruits of M. citrifolia are larger that those of the other Morinda species and are foetid-smelling when mature. The trees have a similar root structure to citrus and coffee, with an extensive lateral root system coupled with a deep taproot.
Morinda citrifolia will tolerate a wide range of climates from dry or humid tropical to subtropical. It prefers temperatures in the range 20 to 35°C, but can tolerate a minimum temperature of 5°C (Janick and Paull 2008, 766). It is also tolerant of harsh environments and saline soils, growing wild from sea level up to an altitude of 500m. Although it commonly grows along seashores, it thrives in many different habitats: low-elevation lava flows, rocky coasts, coral outcrops, brackish tidal pools, open grasslands and lowlands, gulches and cliffs (Razafimandimbison et al 2010). It is also drought-resistant, and can easily withstand a drought of six months or more.
There are three widely recognised varieties of M. citrifolia, the first two already distinguished by Rumphius:
- M. citrifolia var. citrifolia is mainly large-fruited and is pan-tropical, found from Africa to Central America and the Caribbean. It is morphologically diverse, but has no well-defined sub-populations apart from a small-fruited strain that is only found in Micronesia
- M. citrifolia var. bracteata with small fruits and well-developed bracts (small leaves below the fruit), found from India to Australia and the Pacific, including Indonesia
- M. citrifolia var. potteri with small fruits and narrow variegated leaves, restricted to the Pacific.
The large-fruited M. citrifolia var. citrifolia has evolved bisexual flowers and is therefore self-pollinating, flowering and fruiting throughout the year.
The seventeenth century botanist Rumphius listed two species of local morinda on Ambon, which he described as Bancudus latifolia (159, plate 99) and Bancudus angustifolia (158, plate 98). He claimed the first was a shorter ‘female’ species that grew close to inhabited places and was used medicinally, while the second was a ‘male’ wild variety that grew close to the seacoast and was preferred for dyeing (Heringa 1989, 118). The coastal variety has been subsequently identified as M. citrifolia var. braceata (Burkill et al 1966, 1517).

Bancudus latifolia (G. E. Rumpf 1743. Herbarium amboinese, vol. 3, 159)
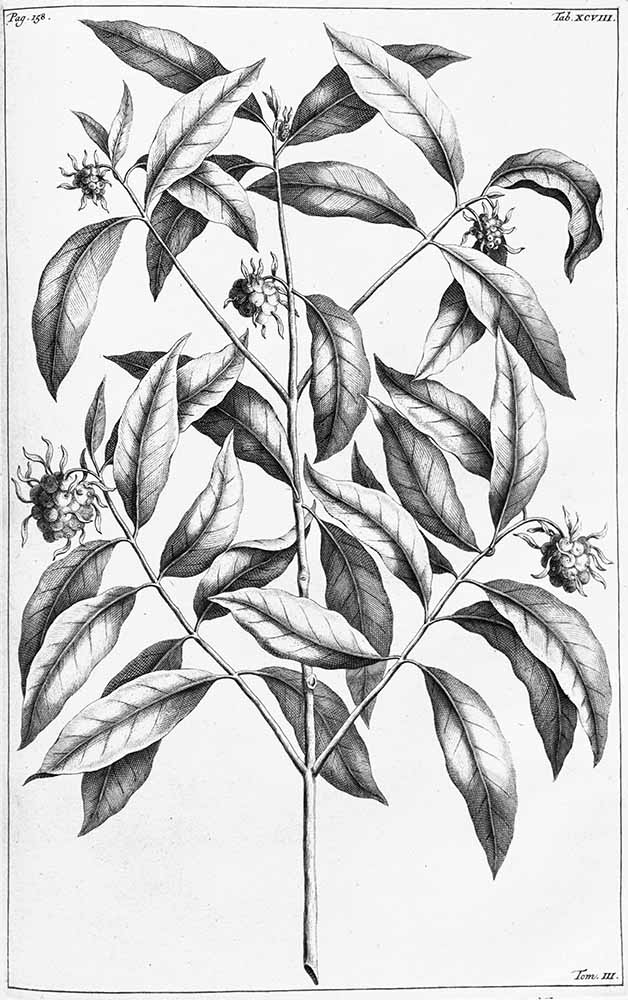
Bancudus angustifolia (G. E. Rumpf 1743. Herbarium amboinese, vol. 3, 158)
Rumphius described how the residents of Ambon produced a colourful stable dye by taking the bark from the thickest roots of M. citrifolia var. braceata and mixing it with a third part of leha leaves and bark (Symplocos) or a little alum (Heyne 1917, vol. 4, 208). Merchants occasionally shipped this Ambonese morinda to Java. Apparently the Malays and Javanese made an even better red dye by mixing the root bark with sappan or other red dyewoods.
The large-fruited variety of M. citrifolia var. citrifolia is preferred for medicinal uses. It is frequently referred to as noni, especially throughout Polynesia. The small-fruited M. citrifolia var. bracteata is preferred as a dye source.
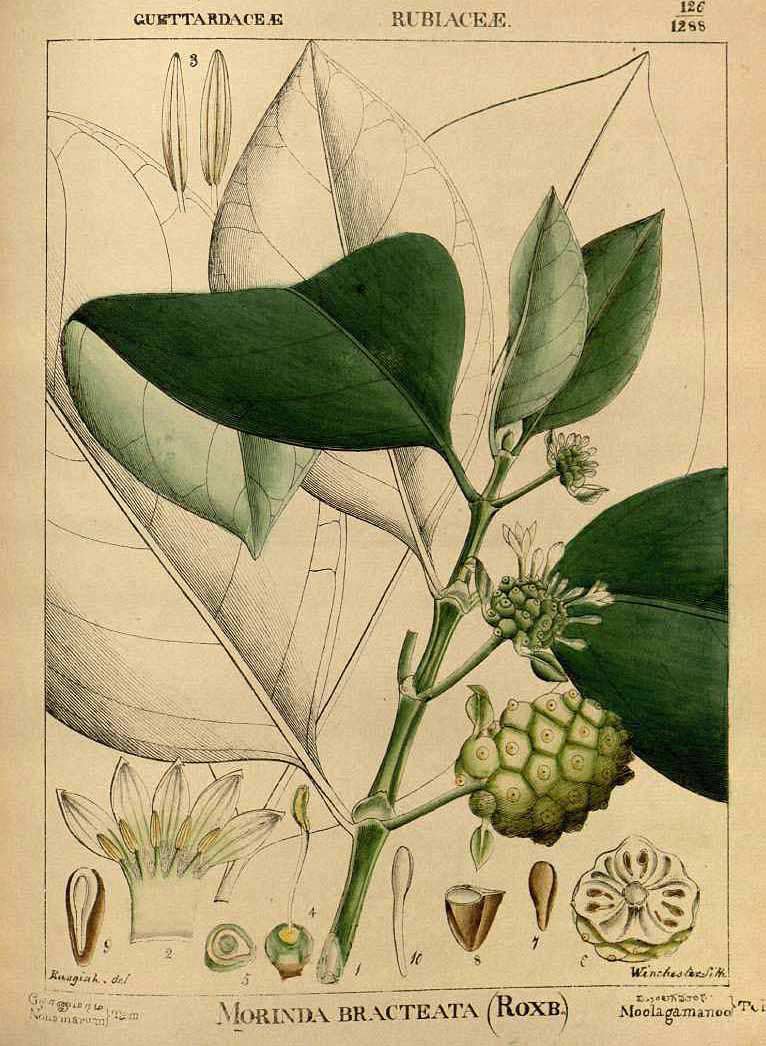
M. citrifolia var. bracteata (Robert Wight 1850. Illustrations of Indian Botany, vol. 2, fig. 126)
The Biogeography of Morinda
Because the large fruited species of M. citrifolia var. citrifolia is distributed globally around the tropics, it has been proposed that it was the ancestor of the numerous regional endemics of Morinda, which are geographically localised or island-specific (McClatchey 2003).
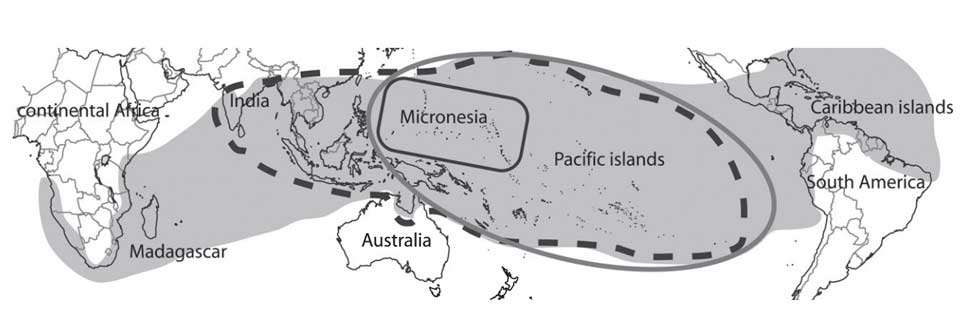
The grey area denotes the geographical distribution of all varieties of M. citrifolia; the black dashed line shows the distribution of M. citrifolia var. bracteata; the grey line that of M. citrifolia var. potteri; and the black line that of the Micronesian small-fruited M. citrifolia var. citrifolia. (Razafimandimbison et al 2010).
A recent DNA analysis has disproved this hypothesis (Razafimandimbison et al 2010). M. citrifolia var. citrifolia is derived from a large genetic Asian clade of Morinda species, which are quite distinct from the more localised species found in Australia, the Pacific, Africa and Madagascar.
The flowers and fruit of M. citrifolia (Baillon, H. E., 1877-80. Histoire des Plantes, vol. 7, p. 292)
In the late nineteenth century the botanist Henry Ridley suggested that M. citrifolia had been introduced into the Malay region from the islands of Maluku (Keng and Keng 1990, 158). However the above genetic study suggests that M. citrifolia originated in Micronesia. Its subsequent widespread distribution appears to be the result of its acquired ability to self-pollinate, its ability to flower and fruit throughout the whole year, and its buoyant fruits - the plant appears to be primarily spread by means of ocean dispersal. Its pungent-smelling fruit quickly decays in seawater to leave a mass of seeds (from 100 to 150), which are very buoyant as a result of their bladder-like cavity. The seeds still remain viable after floating in seawater for several months (Guppy 1917).
One example of its ability to colonise virgin islands is provided by the eruption of Krakatoa in 1883, which eradicated all plant life. After 15 years the island’s beaches had acquired a rich flora, including shrubs of M. citrifolia (Sauer 1988, 21). It has also been found that fruit bats disperse seeds of the large-fruited M. citrifolia inland.
Morinda in India
At least seven different species of Morinda occur in India, the three most common being M. citrifolia, M. umbellata, and M. tinctoria.
M. citrifolia is widespread and was cultivated in the past in Bengal, Orissa, and Burma. It was often allowed to grow as a weed near the homesteads of native weavers who made use of it for dyeing their manufactured fabrics (Dunstan 1903, 209). At that time the roots of the various species of morinda were becoming an increasingly important red dye, gradually supplanting the more expensive chay root. The most common mordants were tannic acid and alum, producing a range of colours varying from reddish-yellow, through pink and various shades of red, to dark brown.
Benjamin Heyne noted that while cotton was dyed with chay root on the Indian coast, the Gentoos of Mysore used M. umbellata, which grew prolifically in the surrounding jungle (Heyne 1814, 91). In the village of Sarti, cotton was first steeped in sesame oil and then immersed in a strong ley of euphorbia ash for four successive nights, being dried in the sun during the day. After washing, the cotton was immersed overnight in a pot of water containing powdered togaru (morinda root) heated over a fire of cow dung. In the morning it was dried in the sun. This cycle was repeated for a further two days. The result was a dirty crimson.
Sir Thomas Wardle studied four species of Indian morinda but struggled to reproduce the colours obtained by Indian dyers. With M. citrifolia he could only obtain yellows and oranges but not red. With M. angustifolia he obtained reddish yellows, brownish reds and dull reds on both silk and cotton (Wardle 1887; Dunstan 1903, 209).
The History of Morinda in Indonesia
The pan-tropical distribution of the dye source M. citrifolia var. bracteata and the medically useful M. citrifolia var. citrifolia suggests that both were present in the Indonesian archipelago well before the arrival of modern humans over 40,000 years ago. It is impossible to say exactly how long morinda has been exploited as a dye, but the use of similar regional terms such as bangkudu, cangkudu, mengkudu and wungkudu across the entire archipelago does suggest that the species has been exploited for a very long time.
Regional names for Morinda |
|||||
Cunningham 2014, 97; Hariana 2008, 118; Hembing 2008, 285; Niessen 2009, 544; Saunders 1997, 93; Verheijen 1984, 61. |
|||||
Sarawak |
kuinbu |
Gorontalo |
bengkudu |
Nagé |
kebo |
Sarawak |
engkudu |
Makassar |
bingkudu |
Ende |
kembo |
Nias |
makudu |
Makassar |
mangkudu |
Sikka |
bur/long bur |
Aceh |
kemudu |
|
|
East Flores |
kelore |
Batak Toba |
bangkudu |
Sunda |
cangkudu |
Solor |
kelore |
Batak Ankola |
bangkudu |
Java |
kemudu/kudu |
Lamalera |
keloré |
Minangkabau |
bingkudu |
Java Central |
pacé |
Roti |
manakudu |
Palembang |
bengkudu |
Java East |
bentis |
Savu |
kèbo |
Mandailing |
pamarai |
|
|
Sumba |
ai kombu |
Lampung |
mekudu |
Madura |
kondhuk |
East Sumba |
kombu |
Kalimantan |
wangkudu |
Madura |
kuduk |
|
|
mangkudu |
Bali Old |
wungkudu |
Uab Meto |
bauk ulu |
|
labanau |
Bali Modern |
tibah |
Amarasi |
uru |
|
rewonang |
Bali Tenganan |
sunti |
Tetun |
ai-nenuk |
|
|
|
Nusa Penida |
sunti |
Fataluku |
nenuka |
|
|
Manggarai |
haju kembo |
Insana |
bengkudu |
Working backwards, linguistic palaeontologists have predicted that the proto-Malayo-Polynesian word for morinda was bankudu (Blust 2009, 667). The controversial ‘out of Taiwan’ hypothesis dates the arrival of proto-MP Austronesians in the Philippines to around 3,500 BC (Blust 1984-85, 46). Linguists have also identified the proto-Oceanic word, nonum, supposedly taken by Austronesian speakers from Indonesia when they migrated into northwest Melanesia around 1,000 years ago (Pawley and Ross 2006, 520).
More reliable historical evidence indicates that morinda dyeing in the form that we know it today was well-established in both Java and Bali by the ninth century AD. Javanese sima charters dating from the early tenth century list taxable commercial activities, one of which was dyeing. One example is the Alasantan inscription of 939 found in East Java. These records show that on Java the two most important dyestuffs at that time were indigo and wungkudu, the latter being the Old Javanese term for morinda (Christie 1993, 186). General peddlers were important middlemen who brought cotton, spun yarn and wungkudu roots to the markets. Also important were the producers of ash, pressers of oil and burners of lime, the latter also for use in betel chewing.
Wungkudu dye processing is also mentioned in Balinese charters dating from the early ninth century onwards. The inscription from the village of Sukawana in Kintamani district, dated 883 AD, exempted local monks from paying taxes on various occupations, including indigo dyeing, mangnila, and morinda dyeing, mamangkudu (Stewart-Fox 1993, 87-88). A second inscription from the village of Bwahan in Kintamani, dated 995, records that wungkudu did not occur in that village. Finally the inscription from Tengkulak close to Ubud, dated 1024, gives the villagers of Songan Tambahan permission to cut down morinda trees, which were otherwise protected. In the twelfth century, trees considered to be of special quality on Bali included wungkudu, jirak (Symplocos fasiculata) and kamiri (candlenut) (Stein Callenfels 1926, 1-6).
When Marco Polo visited Jambi in eastern Sumatra in 1292 he described a tree that may have been morinda, because its roots were taken up for use as a dye (Rhys 1908, 344).
According to Marsden, the morinda tree was known as bangkudu in some districts of Sumatra and mangkudu in others (Marsden 1811, 95). He claimed that the roots of M. umbellata were used for dyeing and that the broader-leaved M. citrifolia did not yield any colouring matter, despite having specified M. citrifolia as a dye plant some years earlier (Marsden 1784, 78). The dried roots were pounded and boiled in alkaline water made from the ash of coconut fruit stalks and leaf midribs. Sometimes the bark or wood of sappan was added to the dye bath. Anderson found that mangkudu root was used as a dye in the Langkat region of east Sumatra, although the trees were mainly planted as a support for pepper vines (Anderson 1826, 249 and 261). The Toba Batak used two types of bangkudu differentiated by region: bangkudu Toba and bangkudu Pahea. The latter was higher quality and therefore more expensive (Jasper and Pirngadie 1912, 69).
Raffles (1817, 38 and 170) found that the two most important dye plants on Java were indigo blue and wong-kúdu scarlet. The dyeing process involved several stages. First the yarn was boiled in an alkaline solution of wijen (sesame) or kamiri oil, the alkali extracted from burnt rice chaff. After drying the yarn was steeped in the wong-kúdu mix, the latter obtained by boiling the bruised roots in water until it was reduced to one-third before the addition of jirak bark (Symplocos). Tronfreville made a few experiments with M. umbellata and considered it a very valuable dyestuff. He noted that it was much used in Java (Dunstan 1903, 210).
According to John Crawfurd (1820, 463), two species of mangkudu (Morinda) were found abundantly in every part of the Indian archipelago, but only the roots of the small-leaved variety (which he described as M. umbellata) were suitable for dyeing. That from the eastern islands was considered superior to that from the western islands, which is why morinda from Ambon was exported to Java.
By 1825 bangkudu was being used extensively in the Goram Islands east of Seram for the dyeing of cotton yarn, used to weave cloth that was exported to the Kei and Aru islands (D. H. Kolff 1840, 307). In the early 1830s George Bennett (1834, 219) discovered that morinda was abundant on St. John’s Island, just south of Singapore, commenting that in the islands of the eastern archipelago (of Indonesia) it was used both as a dye and as a prop for pepper vines as well as a shade for coffee plants. The dye plants M. bracteata and M. tinctoria were reported to be plentiful in the kampongs of Sumbawa (Zollinger 1854, 265). Mangkudu from Java and Sulawesi was even sent to the Great Exhibition held in London in 1851 (Ellis 1851, 880).
Two types of morinda from Buton Island were exhibited at the Brussels Exhibition of 1910 – one called tanah putih was a yellow dye while the other called tanah mérah was a red dye (Heyne 1917, vol. IV, 208). They were both varieties of M. bracteata, the first (mistakenly) thought to grow in deep weathered soils that had no red colouring, the second on reddish rocky shallow soils. Because of its higher dye content, the tanah mérah justified a higher price.
The head of the Museum of Economic Botany at Bogor (formerly Buitenzorg) in West Java confirmed in 1917 that morinda from Ambon was still being exported to Java (Heyne 1917, vol. IV, 207-214). In Maluku the natives used the bark of the thickest roots and mixed them with leha leaves (Symplocos) or alum. However the Malays and Javanese achieved a deeper red by the addition of sappan or some other red bark wood.
On Sumatra morinda is classified as a low altitude beach forest species, growing along sandy coastlines (Laumonier 1997, 129), especially on the west coast. On Java morinda grows wild around the coast and is cultivated at lower elevations inland. In the nineteenth century there were plantations in coastal areas of northern Java and the adjoining islands (Hofmann-de Keijzer and van Bommel 2005, 71). One of the largest plantations was on the Karimonjawa Islands, located to the north of Java (Heyne 1917, vol. IV, 209). Batik workshops at Pekalongan could buy three types of morinda bark in 1912: locally harvested, from Buitenzorg in West Java, or imported from Maluku (Heyne 1917, 211).
It has been suggested that the process of morinda dyeing in Indonesia is of Indian origin, since it was found in textiles with Indian design features (Bühler 1941, 1423-1426). Bühler later suggested that its centre of origin was probably the Coromandel Coast, where it reached its highest perfection, and that the technique became progressively degraded the further it spread eastward, thus explaining why it was so greatly modified in Indonesia (Bühler 1948, 2504). Others have suggested that it was introduced to Indonesia on the back of Indian trade, the Indians having adopted it in turn from the Middle East (Gittinger 1979, 169; Fraser-Lu 1988, 29). It is hard to find evidence for any of this, apart from some weak linguistic links. Firstly the principle natural mordant used in the morinda dyeing process, Symplocos, is termed lodhra in Sanskrit and loba in some but not all parts of Indonesia. Secondly the kingdom of Kediri, which was contemporaneous with the Srivijaya Empire on Sumatra, is thought to have taken its name from khadri, the Sanskrit word for morinda. Based in the Brantas River valley, it controlled much of East Java from 1042 to around 1222. This very same region became one of the main Javanese centres for morinda dyeing in the nineteenth and twentieth centuries.
Unfortunately there has been a widespread tendency to assume that every textile innovation and design used in Indonesia has been imported from Arabia, India, China or Europe. Such ideas imply that the Indonesians were incapable of developing anything original of their own. We need to open our minds to the possibility of unrecorded westwards transfer of agricultural and other technologies developed in Southeast Asia and Indonesia.
Obviously the common Indian name for morinda, aal, is totally different to the Malay terms bengkudu or bangkudu and mengkudu or mangkudu, while the Javanese, who have had more intense trading links with Arabia and India, refer to Symplocos as jirak or jirek. Furthermore the morinda dyeing process in India is considerably different to that found in Eastern Indonesia. Indian dyers frequently used creamy milk instead of oil or alternatively castor or sesame oil; derived their alkali from carbonate of soda or the ashes of plantain; and placed more emphasis on the addition of tannin-rich substances such as plant galls and myrobalan (Napier 1869, 357; Bühler 1948, 2500; Mohanty et al 1987, 11-8). Occasionally they omitted the oiling stage altogether. It seems just as likely to us that the Indonesian morinda-dyeing process could have developed independently, using locally available plant materials.
Morinda in the Lesser Sunda Islands
There has been no systematic island-wide survey of Morinda across the Lesser Sunda Islands, although it has been recorded on specific islands. For example, on Bali morinda has been found to grow in open places close to the sea or in mangroves, while being cultivated on land below 200m in altitude (Heim 2015, 194). On Flores it grows in hot areas close to sea level (Hamilton 1994, 62). However it is not found at higher altitudes, which explains its absence around the weaving villages of Ngada (Hamilton 1994, 66). Meanwhile on Savu, Duggan has identified the presence of two species, samples of which have been identified by botanists at the Bogor Herbarium as M. citrifolia and M. tomentosa (Duggan 2001, 32 and 87). The former is known locally as kebo hida and is the species preferred for dyeing. On islands such as Savu, which have a long dry season, morinda trees sometimes struggle to survive from rainy season to rainy season.
We have observed morinda growing in many coastal regions of the Lesser Sunda Islands. However in some locations, such as Nusa Penida, most of the morinda trees have been cut down for firewood. Morinda has had to be reintroduced and cultivated so that it can once again be used as a dye source.
Morinda grows extensively around the rocky shorelines of Flores Island and even in inland lowland forests such as Dorameli and Wolomeze in Ngada Regency (Russell-Smith, Djoeroemana, Maan and Pandanga 2007; Hidayat and Cahyaningsih 2016). However it will not tolerate high altitude forest. Consequently morinda was not used by the Ngada who previously lived on the higher slopes of Gunung Inierie. To produce their red boku headdresses they therefore used sappanwood rather than morinda (Hamilton 1998, 105). Morinda is however used by the neighbouring Nagé who live around the village of Boawae at the foot of Gunung Ebulobo. Further east it is found in the southern coastal Ende and Lio regions of Ende Regency, on the southern Sikka coast as well as in the regions of Sikka Krowé and ‘Iwang Geté, and around the shores of East Flores, Adonara and Solor.

Morinda citrifolia var. citrifolia growing in Lewoleba town, Lembata Island

Morinda citrifolia growing at the head of a volcanic beach on Ata Dei, Lembata Island
On Lembata morinda grows in gardens around Lewoleba as well as on the rocky shores and few sheltered beaches of Ata Dei. However on Ilé Api the weavers prefer to harvest a local variety of M. citrifolia (presumably var. bracteata), which has smaller fruits and grows in the forests above sea level. At Lamalera the weavers barter for their morinda, which grows wild close to villages located higher up the Ile Labalekan volcano. Only trees growing in certain localities produce good morinda dye. Ruth Barnes (1984, 28) put this down to soil characteristics, but it is more likely related to variety. Today dyers from Ilé Api and Lamalera are being encouraged to set aside land by the government and NGOs for growing dye plants, including morinda. At Watuwawar on the Ata Déi Peninsula, dyers plant morinda trees in their gardens around the village.
In East Sumba local dyers use two types of morinda. In Kambera and Rindi they use kombu, which appears to be M. citrifolia var. bracteata. However in the northern regions of Kanatang and Kapunduk, as well as in the highland region of Umalulu, dyers use kombu ahu (‘dog morinda’), which grows on the dry limestone plateaus. It has smaller leaves, a medium sized fruit and a more yellow-coloured root.

Kombu ahu or dog morinda growing on an inland limestone plateau in East Sumba
On Savu the supply of morinda is very limited and there is a need to plant more trees. Unfortunately many young trees do not survive the long dry season (Duggan, private communication, 2013).
In West Timor the Amfoang and the Amanuban collect their morinda from ‘far away’ or from the forest. The Amarasi, who use morinda intensely in their textiles, have been forced to start a planting programme under the leadership of their Raja, Robert Koroh (Yeager and Jacobson 2002, 65). The Helong at Bolok near to Kupang also have to travel to obtain their morinda from inland forests. Sometimes they uproot small saplings and replant them close to their homes.
It seems that M. citrifolia var. citrifolia grows wild in many coastal regions of the Lesser Sunda Islands, and is mainly used for medicine as well as goat food. However weavers find it harder and harder to find the preferred M. citrifolia var. bracteata, which has a smaller fruit and tends to grow in the hills above the shoreline. There seems to be a process of radial depletion, with weavers travelling further and further afield to obtain their morinda (Cunningham et al 2014, 103).
A recent 2009 survey of 196 natural-dye weavers from 11 kelompok (weavers’ cooperatives) across the Lesser Sunda Islands revealed an emerging problem with the availability of morinda. Weavers were asked to rate their supply of morinda roots at that time on a scale of 1 to 5, where 1 meant ‘There is much more than I need’ and 5 meant ‘There is much less than I need’. The average answer was 3.26, slightly worse than ‘There is enough’ but not as bad as ‘There is a shortage’. For 67% of respondents and 80% of cooperatives (where more than half of a cooperative’s members held the same opinion), morinda supplies were said to have changed over the year before the survey. More than half the members of three cooperatives felt that their supplies had improved, while three others felt they had declined, highlighting regional differences. The principal cause for the decline in supply was cited as overharvesting as a result of the increasing demand for textiles (Cunningham, Ingram et al 2011, 105-106).
Addressing this issue requires not only an increase in the cultivation of morinda in those specific weaving communities affected by shortages, but also better education on how to harvest morinda root bark without destroying the trees. A reliable morinda root-bark drying method is also required, as most users currently use fresh root-bark.
Because of the practice of slash and burn agriculture, large parts of the once widespread tropical and monsoon forests of eastern Indonesia have been converted to savannah (Monk et al 1997). In NTT, forest cover has been recently estimated at just 9.6% of the land area (Kurniawan and Tacconi 2005). A survey of two areas – in Ngada and East Sumba Regencies – has highlighted how unmanaged burning continues to adversely impact forest resources and exacerbates soil erosion (Russell-Smith, Djoeroemana, Mann and Pandanga 2007).
A survey of plant names used in the Lesser Sunda Islands identified M. tomentosa as the species of morinda growing on Flores, Savu, and the other islands (Verheijen 1990, 229). This is clearly incorrect.
The Cultivation and Harvesting of Morinda
Morinda dyewood was normally harvested from trees growing in the wild, but with increasing shortages there has been an increasing attempt at cultivation by both villagers and by local forestry departments across the Lesser Sunda Islands. Morinda can be readily grown from fresh or dried seeds extracted from the ripe and softened syncarps, which turn from green to yellowish-green as they ripen (Nelson 2003). The seeds can be dried and stored or planted immediately after removal from the fruit, ideally in well-drained soil with adequate light. Trees can also be propagated through stem cuttings.

Morinda citrifolia var. citrifolia seedlings
Unlike indigo dyeing, which normally takes place at the end of the rainy season, morinda harvesting and dyeing normally takes place during the dry season when moisture levels in M. citrifolia root-bark are low and anthraquinone levels are high (Cunningham, Ingram, et al 2011, 101). Of course once the root bark has been harvested and dried, it can be used to dye at any time during the year. In the Lamaholot region from East Flores to Lembata, morinda roots were traditionally only harvested at the height of the dry season, in August and September (Barnes 1994, 19). On Lembata, roots are harvested in the second half of the dry season, when the tree is dormant (Barnes 1984, 28). This also allows the tree to recover with the onset of the rainy season.
We have observed that in some places dyers harvest the fine root twigs but in others they use much coarser roots. Obviously the smaller root twigs contain a higher proportion of bark to wood than the coarse twigs. This explains why in India, the price of fine morinda bark was four times the price of coarse bark (Hunter 1799, 38-39). In Bengal, the concentration of morindin was found to be highest in the roots of trees that were three or four years old. As the trees age further the concentration of morindin falls, becoming no more than a trace in mature trees (Thorpe and Greenall 1887; Saxena and Raja 2014, 41). Today villagers in the Lesser Sunda Islands claim that they only harvest the roots of trees that are at least five-years-old, although dating the age of a young tree is a rather imprecise affair.
Harvesting morinda root is normally men’s work. Once a tree has been selected, the soil around the roots is dug away and the roots are severed. In the past the practice of removing the entire root system has led to a shortage of morinda in some areas such as Savu and West Timor. Yet it has been known for a long time that if only a portion of the roots are removed from a young tree, it is possible to continue harvesting for a further five to twenty years (Holle 1866, 340). Today villagers are encouraged to conserve their morinda trees by removing only one third of the root system, thereby allowing the tree to recover (Yayasan Pecinta Budaya Bebali 2011).

Small pieces of morinda root, East Sumba
The amount of root-bark required is considerable and is far in excess of the amount of Symplocos leaf and bark required (Cunningham, Ingram, et al 2011, 104). The following table estimates the quantities of Symplocos and morinda used by four weavers’ cooperatives (kelompok) producing high-quality, hand-woven, naturally dyed textiles on Flores, extrapolated to estimate the quantities required by the ‘Nusantara’ region (the whole Indonesian archipelago):
Cooperative |
Location |
Morinda |
Symplocos |
Weavers |
| Kelompok Bou Sama Sama | Ende | 5,795 | 77.8 | 23 |
| Sanggar Bliran Sina | Maumere | 16,800 | 12.0 | 50 |
| Ebi Rie | Bajawa | 560 | 5.6 | 13 |
| Kelompok Kapo Kale | Ende | 5,184 | 2.9 | 10 |
| Total | 28,339 | 98.3 | 96 | |
| Total ‘Nusantara’ Region | 268,335 | 930.8 | 909 |
(From Cunningham, Ingram, et al 2011, 105)
It suggests that almost three hundred tonnes of morinda root is currently consumed annually across the entire Indonesian region. In the historical past the level of consumption must have been considerably higher.
When dyers needed a deep morinda shade they had to repeat the dyeing process up to twenty times, which consumed around 120 kg of root per textile. Almost thirty years ago Fraser-Lu estimated that on Flores it could take up to 27 kg of morinda root to produce enough dye for one sarong (1988, 30).
Symplocos and Other Natural Aluminium-Rich Mordants
Morinda is an adjective dye – to be effectively used as a dye bark, morinda requires the addition of a metal mordant. In Java and the Lesser Sunda Islands this was, and still is, predominantly achieved by the addition of the leaf and/or bark of trees belonging to the genus Symplocos, both of which are rich in aluminium.
As early as 1700 Georg Rumphius had identified the ‘Aluyn-Boom’ or alum tree (to which he assigned the binomial Arbor aluminosa). Its leaf and bark extract was used instead of aluminium salts as a mordant for dyeing morinda and sappan on Ambon, even being exported for that purpose to the Coromandel coast (Herbarii Amboinensis, book 5, 1743, 160). João de Loureiro confirmed his observations in Indochina in the late eighteenth century (Bühler 1948, 2497). However the importance of these findings seems to have been overlooked until the early twentieth century when Felix Driessen of Leiden discovered that soluble aluminium salts occur in the bark and trees of certain plants. In 1906 W. Rothert quantitatively analysed aluminium in 260 different plants – principally club mosses, tree ferns and nearly all species of Symplocos (Rothert 1906, 43-52).
The genus Symplocos contains 318 recognised species of evergreen flowering shrubs and small- to medium-sized trees (Fritsch et al 2008). They have leathery leaves and produce a low-density timber that is generally unsuitable for construction. In the Old World Symplocos is primarily confined to the eastern part of Asia. It is found no further west than the Deccan peninsula of India but extends eastwards into the Pacific, reaching as far north as northern China and Japan and as far south as New South Wales in Australia (Nooteboom 2013, 447). In the New world it is found from the southeast of the USA to southern Brazil. The genus appears to have originated in Asia and only later dispersed to America (Wang et al 2004).
An early taxonomic analysis of 277 species identified four sub-genera: Symplocos (76 species), Epigenia (20), Hopea, (167) and Microsymplocos (14) (Brand 1901). Despite numerous subsequent reclassifications, these four sub-genera are still recognised today.
Symplocos cochinchinenis var. laurina formerly Symplocos spicata
(Robert Wight 1850. Illustrations of Indian Botany, vol. 2, fig. 150)
Symplocos grows in the warm temperate and tropical climate zones, preferably in moist to wet mixed, mostly evergreen rainforests. It avoids arid zones and is most abundant in mountain forests, generally preferring altitudes from 600m up to 2,000m. However in some regions it has been found at lower levels, even down to sea level. In India, Burma and Thailand it rarely grows at lower levels, preferring hills and mountains up to 1,700m (Nooteboom 1975, 153). In China it grows up to 2,000m and in the Malay Peninsula from sea-level up to 1,300m. In Indonesia it grows in the hills and mountains of Sumatra up to 2,300m, on Java up to 1,600m, on Borneo from perhaps 1,000 to 1,700m, and in New Guinea at altitudes up to 3,000m (Nooteboom 1975, 153). Dwarf varieties have been found up to an altitude of 4,000m on Mount Kinabalu, Borneo, and in the high mountains of Papua (Nooteboom 1975, 36).
Location |
Generic Local Names |
| Malaysia | kerenang, nasi-nasi, menasi |
| Sumatra | kayu loba-loba, djarak bulau, djirok, kekatja, lelebah, pipi udan, lebomelukut, hapu-hapu, havu-havu |
| Borneo | giak, girak, gumiting puteh, idabo, jirah, jirak, labah, loboh, njam-njam, pachal ambok, periaboh |
There are many generic names for Symplocos in Malaysia and Western Indonesia (asianplant.net)
In India, Symplocos has been widely used as a medicine since the first millennium BC, and probably earlier, being detailed in the Ayurverdic texts for gastric and gynaecological disorders, wound treatment and multiple other ailments. In Sanskrit it was termed lodhra, rodhra, tirita or tilaka, in the latter case because it was used for making the tilaka mark on the forehead (Bhusnar, Nagore and Nipanikar 2014). Other regional names include lodha (Hindi and Bengali), lodh (Urdu), mugam (Assamese), lodhar (Punjabi) and kath (Kashmiri). In certain parts of India the leaves and bark were used as a mordant for madder and as a minor yellow dye (Stewart 1874, 299). The dried leaves were even exported from Sikkim to be used as a yellow dye in Tibet (Balfour 1873, 665).
Most species of Symplocos contain high concentrations of aluminium and flavonoids such as yellow quercetin (Nooteboom 1975, 153). One survey of 142 Symplocos species found that 141 qualified as aluminium hyper-accumulators (Jansen et al 2002). Members of the sub-genus Hopea seem to contain the highest aluminium concentrations and their leaves turn yellow as they dry, apparently due to the formation of aluminium-flavonoid complexes. As a note of caution, Nooteboom claims that no accumulation of aluminium has been observed in the Symplocos sub-genus Symplocos, whose leaves never turn yellow (Nooteboom 2013, 446).
Aluminium is the third most abundant element in the earth’s crust and accumulators such as Symplocos appear to have evolved a mechanism to detoxify the aluminium absorbed from highly acidic soils and accumulated in their tissues. The aluminium is made harmless by locking it away in the form of stable organic or inorganic metal complexes (Maejima et al 2014). Indeed the Symplocos species accumulate so much aluminium that they are referred to as hyper-accumulators. Concentrations of between 0.05 and 4.2% of the weight of the dry leaves have been mentioned in the literature (Nooteboom 1975, 19). For example a recent study found aluminium concentrations of 0.8% in the leaves of S. chinensis (Maejima et al 2014). However the species S. spicata, now known as S. cochinchinenis var. laurina, seems to be one of the best hyper-accumulators – its leaves were found to contain over 72,000mg of aluminium per kilogramme – over 7.2% by weight (von Faber 1925). A later analysis of the same species measured a concentration of 7.1% (Webb 1954). Meanwhile the ash of S. tinctoria has been reported to contain over 50% of aluminium by weight (Lepp 2012, 217).


Bundles of loba bark and leaf supplied from the market at Waingapu, East Sumba
Interestingly analysts at the Royal Botanic Gardens, Kew, have found the concentration of aluminium in the old fallen leaves of Symplocos from Flores, Indonesia, (S. cochinchinensis?) to be over 3% by weight, higher than that in the fresh leaves, the bark, or the wood (Goodman, Garcia and Ingram 2013). This has important implications for the future harvesting of Symplocos.
It seems that all of the species used for dyeing belong to the sub-genus Hopea, the one that contains the most efficient hyper-accumulators. The five most important are listed below, along with some of their local Malaysian and Indonesian names:
|
S. fasciculata |
S. cochinchinensis |
S. cochinchinensis |
S. lucida |
S. adenophyla |
Malaysia |
merpadi paya, nasi-nasi, pokok lukut |
medang hitam |
pokok api-api |
|
mendong, menugan |
Sumatra |
kaju loba-loba |
|
|
kayu hotir |
kayu lattan, kayu porugis |
W Kalimantan |
|
|
|
|
kayu kain |
Sarawak |
|
|
|
|
jirak |
Javanese |
jirek |
jirak sapi |
jirek |
jirek |
|
Sundanese |
jirak |
jirak sapi |
jirak, jirak sasah |
jirak lulub |
|
Philippines Ifugao |
|
tabu |
|
|
|
Vernacular names of Symplocos sub-species (Nooteboom 1991)
In the past the inner bark of S. cochinchinensis var. cochinchinensis and S. fasciculata was often used as a mordant in the batik industry and, mixed with other plants, as a dye. It gives a yellow colour by itself, but is more frequently used in the preparation of reds derived from Morinda spp., Caesalpinia sappan, Butea spp., and other dye plants (Nooteboom 2013, 447). In the Lesser Sunda Islands they also use the leaf. In some places Symplocos cochinchinensis ssp. Laurina var. laurina and Symplocos lucida seem to have been used instead. Symplocos adenophylla var. adenophylla was used by the Dayaks of Borneo for dyeing rattan, rather than cotton with morinda (Gavin 2004, 61).
Waingapu-born Hans Nooteboom found that Symplocos grew throughout the Indonesian archipelago, with the largest number of varieties found in Borneo, Malaysia and Sumatra and the least across the Lesser Sunda Islands (Nooteboom 1975). Many of these islands were surveyed between 2005 and 2010 by a small team of natural dyeing specialists who identified the sources of Symplocos used by local weavers and dyers (Cunningham, Maduarta, et al 2011).
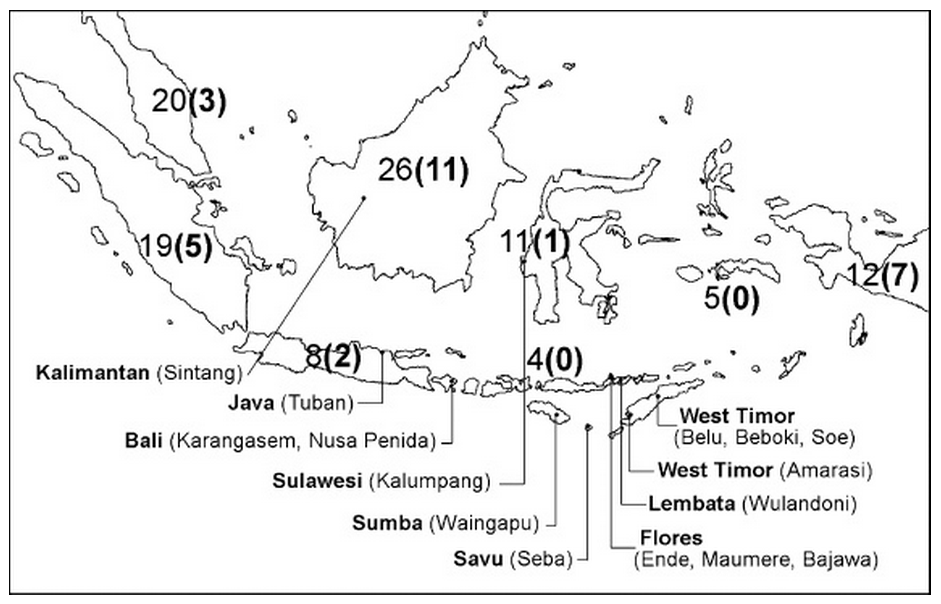
Map of survey sites along with the number of species of Symplocos in each island or island group, the number of endemic species being shown in brackets
(Cunningham, Maduarta, et al 2011, and Nooteboom 1975)
They found that today many Symplocos stands were badly damaged, partly due to deforestation but also because the harvesting of the bark had killed many trees. This destruction may explain why the local price of Symplocos had more than tripled in the five years prior to 2011 (Cunningham, Ingram, et al 2011, 102). The largest accessible stands were found along the high ridges of the volcanic mountains that run along the spine of Flores Island, many of which have been locally protected by customary law. However it does not seem to grow in less mountainous East Flores. Here weavers obtain their lou from merchants from Maumere who in turn source it from the Lio region of Ende Regency (Graham 1998, 234).
On Lembata, Symplocos is not found on the slopes of Ilé Api and local dyers have to buy it from fishermen who bring it over from Alor Island. They call it koka, which means bark. The suppliers keep the nature and location of the koka bark a secret to protect their market. Symplocos does grow on Ile Labalekan above Lamalera. On non-volcanic Sumba it is only found in the Masu Mountains in the Tabundung and Masu-Karera regions in the south of East Sumba. A recent study on Timor found that only a few remnant populations of Symplocos still survived in the montane woodlands of Eucalyptus. These trees were so isolated that very few weavers had ever seen them (Cunningham, Kadati, et al 2014).

A bowl of koka from Alor next to a bowl of morinda root, Ile Api, Lembata Island
There have been two outcomes from the 2011 Symplocos study. Firstly the Bebali Foundation set up the Plant Mordant Project as a means of educating local dyers about the best methods for harvesting, using and conserving Symplocos. Because fallen leaves contain the highest aluminium content, there is really no need to remove bark or fresh leaves from healthy trees. Secondly, women living in two mountain villages in Ende Regency have been supported to start up a small Symplocos supply business. They harvest fallen leaves towards the end of the dry season between August and December and then dry and crush them to make saleable Symplocos leaf powder.

Imported Symplocos on Savu Island
Obviously Symplocos does not grow on the low-lying limestone islands of Semaru, Roti and Savu. Past attempts to grow Symplocos on dry Savu have failed (Duggan 2001, 87). Consequently the leaf and bark has always been imported from Flores or Sumba (Duggan 2001, 32). In the recent past it became unavailable on Savu, forcing local dyers to turn to synthetic alternatives. Thanks to the assistance of Geneviève Duggan, a new source of Symplocos was identified and imports resumed.
Location |
Local Name |
Source |
| Ende-Lio | lobha | Al Khatab 2011 |
| Ndona, Ende | loba | Local weavers |
| Lio, Ende | loba manu | Cunningham, Maduarta, et al 2011 |
| East Flores | lou | Local weavers |
| Flores | lobha | Hamilton 1994, 270 |
| Solor | roman | Barnes 1989, 29 |
| Lembata, Ile Api | koka | Local weavers |
| Lembata, Lamalera | loba, romlolo | Cunningham, Maduarta, et al 2011 |
| Uab Meto, West Timor | nobah | Cunningham, Kadati et al 2014 |
| Tetun, West Timor | usu kain, us’kain | Cunningham, Kadati et al 2014 |
| Bolok, West Timor | loba | Local weavers |
| Savu | luba | Duggan 2001, 32 |
| East Sumba | lobha wawi, luaba | Cunningham, Maduarta, et al 2011 |
| Maluku | leha | Heyne 1917, vol.IV, 207-214 |
Local generic names for Symplocos in the Lesser Sunda Islands
The most common substitute for Symplocos is Aporosa, a genus of about 80 species of small trees that are primarily confined to the rain forests of Southeast Asia and Indonesia (Schot 1988). In the past Aporosa frutescens was imported into West Java from Sumatra and Bantam Island for use in the batik industry of Batavia (Steinmann 1947, 2098). On Java it was known as sassah (Pamphlets on Biology vol. 2958, 134). Aporosa frutescens was widely used by the Iban, who referred to the tree as the jangau or janggau. They collected the bark in the forest and then chopped and ground it into a powder before adding it to the engkudu dyebath (Gavin 2004, 61; Fujisawa and Nakashizuka 2012). Aporosa bark was also use by dyers on Roti, who lacked a local source of Symplocos (Bühler 1948, 2499).
In Tenganan on Bali morinda dyers used the bark of the tropical fruiting tree menteng (Baccaurea racemosa), also known as kepundung or mundung on Java (Cunningham, Maduarta, et al 2011). The bark was also used in Malaysia as one of the ingredients in the pecan dye process, used to colour silk yellow, red or mauve (PROSEA).
Although Symplocos grows prolifically in the mountains of Borneo, dyers in Kalimantan preferred to use a variety of other species as their source of aluminium for mordanting cotton: the bark of trees belonging to the Xanthophyllum species, the seeds of the trees Scleropyrum wallichianum, Hydnocarpus sp. and Elateriospermum tapos, and nuts from the climber Hodgsonia macrocarpa (Cunningham, Maduarta, et al 2011).
On the coasts of Malabar and Coromandel in India it was traditional to use the leaves of casha (Memecylon tinctorium) as the source of aluminium for mordanting chay root (Driessen 1902, 164).
Sources of Vegetable Oil
The initial mordanting stage for the dyeing of morinda involves soaking the cotton yarns in a naturally-derived unsaturated oil, normally extracted from nuts or seeds. After a long immersion it is usual to add some form of alkali to assist lipolytic enzymes within the mix to hydrolyse the triacylglycerols that make up the oil into fatty acids.
In Indonesia, the main sources of vegetable oil are as follows:
The most readily available oilseed in eastern Indonesia is extracted from the nut of the Candlenut tree (Aleurites moluccana), widely known as kemiri (Cunningham, Ingram, et al 2011). It has many other vernacular names such as kembiri in Sumatra, kameri or komeri on Java, and kumeri in Maluku (Krisnawati, Kallio and Kanninen 2011, 1). Candlenut is found in tropical forests up to an altitude of about 1,200m. It grows prolifically in the highlands of Flores, especially in the Ngada and Ende Regencies, where it is harvested commercially, and also in Manggarai, Sikka and East Flores. It is also widespread in Sumatra, Kalimantan, south Sulawesi, Bali, Lombok, Lembata and Alor (Krisnawati, Kallio and Kanninen 2011, 1).

Candlenut trees cover the hillsides of Flores Island, easily identified by their white leaf tips
Most dyers do not harvest the candlenut themselves. For example, dyers at Lamalera obtain it from the nearby Wulandoni barter market. On Flores candlenut is a major cash crop and there is a local cottage-industry harvesting and removing the nuts, which are then sold on to Chinese middlemen. In 2013 the market price was IDR 15,000 per kilo. Even the shells are sold to be used as a biofuel.
Villagers harvest the nuts from the trees or collect them when they fall at the end of the dry season in November. They are spread out on the ground and are sundried in the open air. The whole family sit in the yard together removing the shells using a simple hand-held bamboo tool that holds a single nut so that it can be struck against a flat rock. In some areas, villagers even buy tree seedlings to extend their candlenut plantations for the future.

Removing the hulls from candlenuts at a mountain village in Ngada Regency

Sacks of candlenut shells waiting to be collected by the dealer from Bajawa
Candlenut does not grow on the low-lying limestone islands of Roti and Savu. Here they extract their oil from the seeds of the nitas or Java olive (Sterculia foetida), also known as kalumpang or kelumpang, a tall deciduous tree that tolerates the long dry seasons of these islands. The seed husks are sometimes burnt to produce ash for the making of alkali.

A nitas tree with open seed pods, Savu Island
Another source of oil in the Lesser Sunda Islands is the nut of the tall klewek tree (Pangium edule), also known as kluwak or kluak, which grows in the mangrove swamps. It is called pangi in Bali and Sulawesi, and kapayang or kepayang in Malaysia, Sarawak, Kalimantan and Sumatra. The seeds contain cyanide and are also used to make fish poison. Kapayang oil is the preferred mordant used by the Dayaks of Sarawak and Kalimantan (Haddon 1936, 21; Low 2008, 155).
Less common is the use of oil from the seeds of the kasambi tree (Schleichera oleosa), also known as kesambi, kosambi, kusambi or kahembi (Quattrocchi 2012, 3359). In India it is known as the lac or kusum tree. This species is particularly widespread in the dry woodlands and deciduous forests of eastern Indonesia, where it is widely used for firewood and for the production of lac resin, especially on Sumba (Cunningham, Ingram, et al 2011). It also produces edible lychee-like fruit. Kusambi oil is also known as Macassar oil.
Less common sources of oil include beans from jarak, the Castor Oil shrub or small tree (Ricinus communis), seeds from the Sandalwood tree (Scleropyrum wallichianum), and finally coconut.
The Natural Morinda Dyeing Process
Morindone is an adjective dye that does not adhere to cotton without a mordant, a chemical intermediate that binds the dye molecule to the cellulose polymer. However most metallic mordants, such as alum, do not have an affinity for cellulose and are easily washed away.
The solution is to pre-treat the cotton with a mixture of fatty acids derived from the oil-alkali soaking so that it becomes receptive to the morinda combined with the aluminium mordant. Another is to add tannins, which bind strongly to the cellulose surface and readily form complexes with metallic mordants like aluminium.
In the Lesser Sunda Islands it is traditional to use a mixture of:
- vegetable oil extracted from nuts, seeds, beans, or coconut flesh
- alkali from wood ash, or from lime obtained by burning coral or sea shells, and
- vegetation that is hyper-rich in aluminium, especially the leaf and bark of the loba tree (Symplocos sp.)
After harvesting, the morinda roots are cleaned and the outer bark is removed and dried. However in Lamalera some dyers are now using fresh morinda root to produce a brighter red.

Stripping the bark from morinda roots, Prailiu, East Sumba. The wasted root is used for firewood
The specific protocol for dyeing with morinda differs from weaver to weaver (and is often kept secret) but the following outline illustrates the general procedure.
Prior to dyeing the cotton yarns are cleaned and pre-treated. First they are washed in alkaline water obtained by filtering it through a basket packed with wood ash. Often the water is boiled. This washing not only removes dirt, but also scours away the protective waxes and pectins contained within the outer cuticle of the fibres. Dyers prefer to produce the ash for this process from certain local materials such as the shells of nitas nuts, or wood from either tamarind or kasambi trees. After drying the yarns look quite different. They are softer, whiter and have lost their greasy appearance.
In some cases this initial cleaning process is not required because the yarns have already been cleaned and have completed the first dyeing cycle with indigo.
The yarns must now be oiled in a solution of alkaline wood ash water to which has been added the oil or the crushed seeds, beans or nuts of the chosen oil plant. Substances such as rotten candlenut or rancid coconut are sometimes preferred, being claimed to have a higher content of oil. This is not so – the difference is that the rancid oil has already been partially hydrolysed into fatty acids and smelly aldehydes. Some dyers add small amounts of animal fat and other local plants, including some that contain tannin. It is probable that enzymes within this mixture break down some of the oil and unsaturated fats into fatty acids, some of which are then saponified by the alkali to make them water soluble, a process akin to making soap. After oiling, the yarns are thoroughly dried and the process is repeated. At the end of the oiling process the yarns are washed to remove any excess oil.

Rotten candlenut, East Sumba
After thorough drying the cotton can now be dyed with the morinda bark. The dried chipped morinda root is wetted and laboriously pounded into a pulp, which is then soaked in water before being pressed or wrung to extract the morindin. The squeezed pulp is then pounded again, returned to the dye bath and wrung out again. This is repeated four or five times until all of the morindin has been extracted from the pulp. In some cases the dried aluminium-rich loba leaf and bark is added to the pounded morinda pulp. In other cases dry powdered loba is added later to the muddy-brown morinda soup. Following the addition of lime, the brown dye bath instantly turns reddish-brown as the glycoside morindin is hydrolysed into the red morindone pigment. Some dyers also add tannin-containing plants such as tamarind and other ingredients to the pulped morinda root.

The master weaver Theodora Gelu from Lamalera holds a skein of cotton that has been immersed just once in morinda for two or three nights. The undyed skein in the middle is untreated while that on the right has been boiled in ash water
Morindone is a relatively weak dye - one single immersion produces only a pale pink colour. However the longer the morinda and mordant impregnated threads are left to dry, the stronger the colour produced. After more morinda pulp is added to the bath, the cycle is repeated. Dyers repeat this process over a period of days, weeks, months, and even years, hanging the threads in the sun to fully dry in between each immersion. Sometimes the partially dyed threads were stored away until the next dyeing season. Yarn for the finest cloths only achieves the required deep morinda red after repeated dyeing over many years. Today few dyers have the patience to prolong this process as they did in the past.
Morinda Dyeing in Indonesia
In Sarawak and Kalimantan the Dayak ritual mordanting process for morinda, engkudu, is called ngar or nakar ubong (Haddon 1936, 21; Linggi 1999, 33). Ngar was considered the most important stage in the production of ritual cloth, surrounded by numerous ritual restrictions. It was a group event and its leader was accorded the highest prestige available to a weaver. In Sarawak the ceremony for conducting the process is called kayau indu or the women’s warpath (Ong 1996, 23). Only a few experienced weavers had the knowledge to do this, which was inherited from their mothers. They bore special ritual tattoos and were given the status indu tau’ nakar tau’ ngar or ‘she who knows the secret of measuring out the ingredients in order to obtain the rich colour’ (Gavin 1991, 4; Ong 1996, 106). In the past they were honoured by being the first to receive the pua kumbu containing the newly-taken trophy skulls from the returning headhunters (Sather 2006, 98).
The most common oil used on Borneo was extracted from the nut of the kapayang tree, which thrived in the coastal mangroves and swampy forests (Haddon 1936, 21; Low 2008, 155). This was mixed with a cocktail of fruits and pounded ginger. Apparently many other oils were also used, such as from the kerampai nut (Elateriospermum tapos), the seeds of the woody rainforest climber kepayang akar (Hodgsonia macrocarpa), Gonocaryum calleryanum, and Scleropyrum wallichianum (Cunningham, Maduarta, et al 2011). Sometimes small amounts of animal fat were added to the concoction. The Iban Dayaks pre-dyed their warps with yellow. The source of the aluminium mordant was always janggau, the powdered bark of Aporosa frutescens, applied in an alkaline solution made from lime or wood ash (Gavin 2004, 57 and 62).
In the Toba Batak region of Sumatra, local Toba bakudu was mashed and mixed with the ashes of sanduduk (Melastoma), a rhododendron-like species that is another aluminium hyper-accumulator (Jasper and Pirngadie 1912, 69). Some pig fat was added to the dye bath and the yarns were immersed every day for a period of four weeks. Sandra Niessen interviewed elderly weavers from the Silindung Valley who remembered boiling dried bangkudu root and then adding ‘senduduk‘ ash and banana, but made no mention of the addition of oil or fat (Niessen 2009, 441). Niessen also mentioned a recipe published in a 1975 thesis by Marbun, in which yarns were soaked in a solution of morinda powder, turmeric, lime and ash. Cow or water buffalo fat was added after the first immersion (Niessen 2009, 441).

Kain batik bangbangan (Jasper and Pirngadie 1916, plate 2)
On Java morinda was extensively used by workshops along the northern coast of East Java. The resulting batiks were called kain bangbangan if they were just red or kain bang-biru, literally red-blue, if they were red and indigo (Jasper and Pirngadie 1916, 43-44).
A variety of different recipes have been published. In 1887 batik makers in Central Java pre-treated their cotton for 15 days in a lye of wood ash containing the oils of jarak (castor oil) and katjang (peanut oil). They then immersed it for 5 days in a paste of powdered morinda root and powdered jirak (Symplocos) in a ratio of 2:1 (Van Musschenbroek 1877, 24-27). A later report claimed that the dyeing process took up to 24 days to achieve a deep red colour. The waxed pre-mordanted cloth was steeped in a bath of morinda and jirak (Symplocos) for six days, after which it was washed in the river and dried. This process was repeated until the required colour was achieved (Jasper and Pirngadie 1916, 44). According to Dyrenforth however, the initial mordanting process could take up to forty days. The cotton cloth was steeped in a mixture of lye from the ashes of rice straw or wood from banana trees, and oil from groundnuts or castor oil beans, and to a lesser extent from sesame seeds (Dyrenforth 2003, 30). After mordanting the cloth was sized and beaten to make it malleable, and then finally waxed.
Morinda Dyeing in the Lesser Sunda Islands
In the Bali Aga village of Tenganan Pergringsingan, in eastern Bali, they traditionally dyed their cotton with morinda after pre-treating the threads with candlenut oil for a period of 80 days (Bühler 1948, 2499-2500). This was followed by 25 immersions in morinda with intermediate drying times of three months. The Bali Aga believed that it was essential to expose the drying threads to both the sun and the overnight dew. One especially prized shade of red could take up to six years to produce (Hofmann 1996, 7).
The Balinese living south of Tenganan pre-treated their cotton with an aqueous mixture of wood ash, turmeric and coconut oil, kneading the cotton vigorously before drying for 24 hours, and repeating the process over and over again. The morinda was mordanted with an unspecified wood bark (Symplocos or Baccaurea?), the threads being left in the dye bath overnight before being dried for at least three full days. This process was repeated up to 30 times for especially good results, the threads being left to dry for up to 15 days between immersions (Bühler 1948, 2500).
On Flores morinda dyeing is confined to the central and eastern parts of the island. There is no tradition of using morinda in Manggarai, and it was never used by the Ngada who previously lived in high elevation villages on the slopes of Gunung Inierie (Hamilton 1994, 66). For red edgings and head cloths the Ngada used sappanwood mordanted with lobha leaves. However morinda was used for plain warp stripes in the indigo ikats of the Nagé living around Gunung Ebu Lobo.
The Lio of Ende Regency employed morinda extensively in the past, using a lye made from wood ash, crushed candlenut and powdered lobha (Hamilton 1994, 65). After immersing their threads in morinda up to ten times, some dyers finished them in a hot solution of sappan wood to deepen the red into a distinctive earthy brown. Because morinda dye takes time to bond with the fibres, dyers in Nggela would re-dye their yarns year after year to build up the colour. These yarns were then stored away and were eventually woven many years later. Very few naturally dyed lawo are produced in Nggela today, but those that are still take at least two years to produce because of the length of the morinda dyeing process (De Jong 2013, 269).
By the early 1950s villages close to Ende had already switched to synthetic dyes (Kennedy 1953, 23). Today dyers in the coastal weaving villages of Wolotopo, Ngala Upolo, Jopu, Wolojita, and Pora rely exclusively on chemical dyes. Even in the prestigious village of Nggela, the suspicion is that many dyers have learnt how to cleverly combine natural and synthetic dyes to mimic the appearance of naturally dyed cloths (De Jong 1994, 214). The weavers promote the idea that they still depend on natural dyes by demonstrating the morinda dyeing process to visiting guests.

Bound morinda-dyed skeins drying in the sun at the hamlet of Onelako
Fortunately the tradition of using morinda is still retained in the neighbouring hamlets of Onelako and Manulondo in Ndona District, close to Ende City. In this area they do not use pulverised candlenut to oil their yarns but pressed candlenut oil from a plastic water bottle. The oil is added to ash water mixed with crushed turmeric and the pounded leaves of two local plants, ngaga mbi and moke tahi. After immersing their yarns in the candlenut lye, they remove them and beat them with a stick to ensure the oil penetrates all the fibres (Hamilton 1994, 65). They pound the bark of the morinda roots with more local plant materials and the leaves and bark of lobha harvested from the forests above Ende. The oiled yarns are added to the warm morinda dye bath and left overnight, after which they are washing in the river. This process is continued a number of times and then the cycle is repeated over again using a hot dye bath. Jackfruit leaves are added to the mix for the final immersion to deepen the reddish-brown colour. In total, the number of morinda immersions is around eight.
Some dyers in Onelako and Manulondo finish the morinda dyeing process with one final immersion in a hot dye bath made by boiling sappanwood, known locally as usuk. However sappanwood is not used by the master weaver Sisilia Si'i and her daughter Graciana.
Ende dyers similarly complete their morinda dyeing with a final hot dye process called jaka, in which the red morinda is overdyed to create a browner red. The bath is prepared from the sliced roots of sappan and morinda mixed with Symplocos leaf powder (Cunningham, Maduarta, et al 2011).
Surprisingly little has been written about the past use of morinda in Sikka Regency, where it is valued as an essential requirement for their bridewealth cloths. It seems to have been traditional to add tannin-containing plants such as tamarind and various tree barks to the dye bath. Some dyers in the Maumere region added the bark of a small tree known as rawamatan (Gomphandra mappioides). We suspect this is also a source of tannin rather than aluminium (Cunningham, Ingram, et al 2011). Today dyers at Doka in the ‘Iwang Geté highlands add mango bark and jackfruit bark to the morinda root bark to get a better colour. At neighbouring Watublapi they add three different tree barks.

Women at Sikka dyeing skeins of cotton, probably morinda, in wooden troughs
(Ethnologica, vol. 5, Leipzig, 1941)
The bridewealth cloths of the Lamaholot are also dominated by morinda, both on its own and dyed over indigo. In East Flores it could take up to ten years to complete the morinda dyeing cycle (Bühler 1948, 2487). At Ilé Mandiri and Loba Tobi, where morinda was respectively termed kĕrora and kĕbukä, the root bark was dried and crushed between stones and then mixed with the grated bark or crushed leaves of a tree called roma, presumably Symplocos (Vatter 1932, 220). The yarns were immersed for two days. In the Léwoléma region of East Flores, dyers added gambir (known locally as gamé) to the morinda dye bath, as well as alkali obtained from the ashes of ironwood bark (Graham 1994, 234).
At Lamalera on Lembata, Barnes found that the morinda dyeing process was very simple and devoid of secret ingredients (Barnes 1989, 29). The yarns were apparently pre-mordanted with oil, alkali and aluminium, although Barnes does not identify the source of aluminium used at that time (today the dyers use loba). Oil was added by vigorously stroking the sized yarns with coconut husks. To prepare the dye the roots were chopped into very small shavings before being mixed with boiling water and lime in a large earthenware pot and were then left overnight to steep. The pre-mordanted yarns were immersed in the cold bath the next day, left for two nights and then dried. More root shavings and a coconut cup of lime were added to the bath and the yarns immersed again. After repeated immersions the partly dyed yarns were set aside for a whole year to allow the dye to bond with the fibres. The yarns would receive more immersions during the following year to darken the colour.
Today dyers still immerse their yarns in the dye bath for two or three nights before drying but usually repeat this less frequently, normally just five or six times over a period of one month.
Around Ilé Ape they mordant their morinda with Symplocos bark imported from Alor, which local dyers refer to as koka. At Napasabok, formerly Mawa, dyers give their yarns an initial immersion in morinda prior to binding, so that any unbound sections will not be pure white. After binding and dyeing with indigo, they carry out seven more cycles of morinda dyeing, allowing a fortnight between each immersion for the ikatted yarns to dry. For the finest work, master dyer Monika Kihan immerses her yarns in morinda for two days and then leaves them in her house to thoroughly dry. In total she repeats the morinda dyeing process thirty to forty times.
To obtain a deeper shade of indigo, some dyers in the Ilé Api region pre-dye their yarns with several immersions in morinda before binding the pattern and commencing the initial cycle of indigo dyeing.
In the past, some Amarasi weavers in West Timor completed their dyeing process in the similar way to the Endenese - with an after-treatment of sappan or brazil wood mixed with a tree lichen that probably acted as a source of aluminium (Bühler 1948, 2499). This no longer happens and may explain why the older cloths have a brown tone whereas the new cloths have reddish tones. Today, Amarasi weavers at Baun in Amarasi Barat oil their yarns with a mixture of candlenut and a local green tree leaf called utah ruhna. The two ingredients are pounded in a mortar and the contents squeeezed into a greenish coloured ball and added to water. The ikatted yarns are immersed in the mixture from 4 to 7 days before being thoroughly dried. Apparently some dyers use kusambi rather than candlenut (Usif Robert Koroh 2016, personal communication).

Fresh utah ruhna leaves and balls of candlenut pounded with the same green leaf
Baun, Amarasi Barat
Today the remaining ten Helong dyers at Bolok, in Kecematan Kupang Barat, oil their yarns with pulped candlenut that has been mixed with the inner bark of derus and the leaves and bark of the liti or litti tree. Derus, known elsewhere as dadap (Erythrina species), is a deciduous medium-sized tree with red flowers, which is used to shade coffee plants. However on the dry stoney ground around Bolok it only grows 2 to 3 metres in height. Litti is a medium-sized tree that is similar to kapok. The dyers say that it is like a softener - if they do not use litti, the dyed yarns will be stiff and hard.

A Helong family preparing morinda next to branches of litti leaves, Bolok, Kupang Barat
First the dyers pound the litti in a hardwood mortar and immerse the pulp in water. They then add the pounded derus bark before pounding the candlenut and adding the oily pulp to the mix. They use 200 grammes of candlenut for two skeins of commercial cotton. The oiling solution is bright green in colour. The cotton yarns are added and allowed to to soak for one whole week, after which they are dried for five days. In total they oil the yarns ten times in ten different oiling solutions.

Yarns soaking in the green oiling solution made from candlenut, derus and litti. Bolok, Kupang Barat
For the morinda dye bath they squeeze out the morindin from the wet pounded morinda root and then pound it again several times more. During the final pounding they add some nuts from the nitas tree, which makes the dye bath soapy. They produce their alkali by filtering water through ash obtained by burning the outer shells of nitas nuts (Sterculia foetida). As this is added to the brown morinda bath it turns red. Finally loba powder produced by the Bebali Foundation is added in the ratio of two spoonfuls for every two litres of water. The cotton yarns are immersed in the bath for one week before they are removed and dried. They prefer to leave their morinda bath in the hot sun, and believe that a good mix should bubble. They repeat the morinda dyeing process ten times. At nearby Baun, Amarasi weavers belonging to the Kai Ne'e cooperative immerse their yarns in the morinda bath eight times.

Alkaline ash water being added to the morinda bath, Bolok, Kupang Barat

A bubbling bowl of morinda, Bolok, Kupang Barat
In the Biboki region of North Central Timor Regency dyers currently oil their yarns in a diluted paste made of pulverized candlenuts, sandalwood, and the leaves and bark of the coral tree, Erythrina spp. (Jakarta Post, 11.01.2004).
Apparently some dyers on Timor pre-treated their cloth with candlenut mixed with the leaves of Calotropis gigantea and Datura fastuosa, plus the leaves and bark of other plants (Hoffman 1997). The morinda bath was enriched with a cocktail of additional plant materials – the twigs of the timon tree (Timonius timon), the dark red flower heads of Globe amaranth (Gomphrena globosa), the bark of the bishopwood tree (Bischofia javanica) and the leaves of another unidentified plant (Bühler 1948, 2499; Hofmann 1997).
On Roti, weaving is now predominantly in the hands of the Ndaoese and we have only been able to find one elderly weaver at Sedaoen who still dyes with natural morinda root, harvested close to the village. In the 1930s the cotton was first soaked in a solution of fine ash powder obtained by burning the fruit peel and seeds of nitas (Sterculia foetida) and the bark of Aporosa frutescens. It was left in this mixture for nine days during which time it was vigorously kneaded. After drying in the sun, the cotton was simultaneously mordanted and dyed in the dye bath, which contained the extract of crushed morinda roots and the powdered bark of Aporosa frutescens or Symplocos fasciculata. The threads were vigorously kneaded and sun-dried repeatedly until the required tone was achieved before being finally dipped into a bath of sappan wood, starchy grains of maize, a local orchid (Dendrobium) and the fruit of the spiny herb Solanum indicum (Bühler 1948, 2499).
Morinda dyeing is still very much alive in two hamlets on Savu but no longer takes place on Raijua. On Savu, young morinda saplings are deliberately planted close to the weaving villages and are tendered by hand. Morinda root is harvested from mature trees during the dry season when they are dormant. For oiling local dyers at Ledetadu hamlet use the pounded nuts of local nitas trees (Sterculia foetida) mixed with oyang (Chinese okra or bitter gourd, Luffa acutangula) and papaya leaf. For preparing the morinda dye bath they mix the pounded morinda root with loba imported from either Flores or Sumba. Lime is obtained by burning live coral - each family is allowed to collect live coral for this purpose once a year. A few sprigs of a local herb are added to the dye bath to improve its smell. To achieve a rich reddish-brown the ikatted cotton yarns are immersed in the morinda dye bath every day for one whole month. One interesting local custom is that dyers cannot commence the morinda dyeing stage until the blue stains on their hands obtained during the indigo dyeing stage have faded.
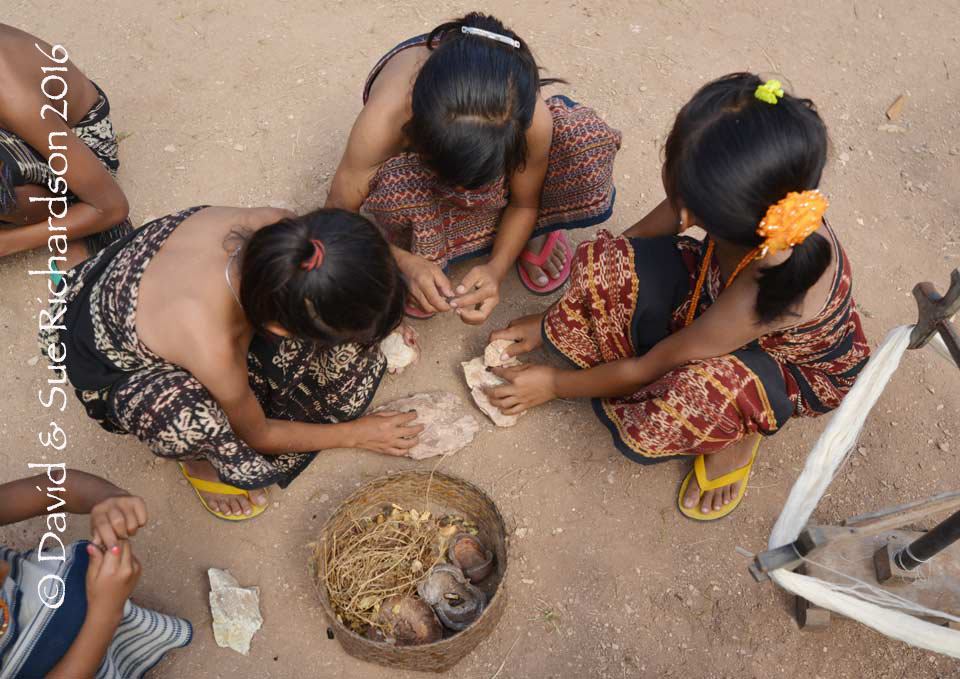
Young girls extracting the kernels from nitas seeds on Savu

Papaya leaf and oyang gourd, Savu

Pulped morinda in a wooden mortar, Savu Island

A pot of morinda dye and a handful of morinda pulp, Savu Island
In East Sumba the majority of red now comes from commercial dyes, but traditional morinda dyeing is still practised in the Prailiu and Mauliru districts of Kambera, and in Pau, Rindi and Kaliuda, but is now rarely found in Kanatang and Kapunduk. In the latter two regions dyers used kombu ahu (‘dog morinda’), which produced a more orange, less intense red. Generally there are more morinda dyers than indigo dyers in East Sumba, the latter being seen as a more specialised family profession. Indeed there are even some male morinda dyers in Parai Yawang, Rindi, who took up the craft following the 1970s boom in Sumbanese ikat.
In the past the morinda dyeing process remained a closely guarded secret. Reverend Louis Onvlee, who lived on Sumba from 1926 to 1947 and from 1951 to 1955 (albeit in Waikabubak), recounted only ever seeing a woman dyeing morinda once, while Bühler could not find a single dyer during his two-month-long sojourn in East Sumba (Adams 1969, 75). Adams discovered that even Dutch expatriates who had lived on the island for twenty years had never witnessed the process. In Kambera, in the 1950s, one noble built a brick wall around his yard to conceal his morinda dyers from prying eyes.
Today dyers are not so shy. Candlenut is universally used for the oil, and dried loba leaf and bark from Masu is used for the metal mordant. Some dyers in Kambera prefer to oil their yarns with rancid waste candlenut, which is black or dark brown in colour and has an awful smell. They pound 1½ kilos of candlenut, add it to five or six litres of water, squeeze the pulp, and filter off the emulsified solution. The candlenut filtrate is then pounded again and added to the liquor to be squeezed again. The indigo-dyed yarns are immersed in the oily emulsion, which contains small fragments of candlenut that stick to the bound bundles of threads. The process is repeated several times, the yarns being allowed to fully dry in the sun for some days between immersions. After the last oiling the yarns are left to thoroughly dry for three months. Once the white yarns begin to turn yellow, they are ready for the morinda dyeing stage. If the oiled yarns are not properly dried, the morinda produces a lighter red (Tamu Rambu Hamu Eti 2016, personal communication).

Bound morinda-dyed yarns drying at Prailiu, East Sumba
Careful drying is also required during the morinda dyeing process. The yarns are steeped in the morinda bath for several days, then wrung out and steeped again before being hung up to dry for six to seven days. This is repeated up to ten times to achieve a deep rust-red tone. After the bindings are removed, the finished cloth is washed in the river. Some of the morinda is washed out of the textile and adheres to the white undyed threads, turning them a light cream.
In Kaliuda morinda dyeing is less common than in the past, and tends to be the specialty of certain families. Several women work together, with two taking turns to pound the wetted morinda and loba in a wooden mortar, while another immerses the fibrous balls of pounded root bark in a tub of water to squeeze out the brown pigment. The latter is then returned to the mortar for more pounding, a process that is repeated four or five times. To ensure that every last bit of dye has been extracted the squeezed ball is finally placed in a small amount of water in a bucket and is wrung out one last time, the resulting solution being added to the main dye bath. Following the addition of alkali, the bath becomes red and is ready to take the pre-oiled yarns.

Women pounding morinda pulp in a mortar, Kaliuda, East Sumba

Squeezing out the morinda pulp, Kaliuda, East Sumba

Indigo and morinda-dyed yarns drying at Kaliuda, East Sumba
The red in older inggings tends to be very similar to the rust-red found in good quality hinggis from Kambera and Rindi. However the red in later examples is a bright scarlet. Certainly a deep red colour is highly valued in this region, and some dyers complete the morinda dyeing process by covering the dyed yarns with a mat and beating them with sticks to ensure that the dye fully penetrates the yarns. The yarns are then placed in a basket and are left in a hot room.
It has been claimed that some dyers achieve a bright red by adding sugarcane to the dye bath, but this cannot be corroborated. We have a suspicion that given the widespread use of synthetic dyes in this region, some dyers enhance the colour of their morinda by the addition of some chemical alizarin red.
Overdyeing with Morinda
As a rule in Indonesia, threads are generally dyed first with indigo and then with morinda. To achieve a dark brown or purple, the indigo threads are normally overdyed with morinda. Thus in Sikka they dye the cotton once or twice with indigo and then overdye as many times as required with morinda. The resulting colour is called wunggung (Lewis 1994, 162). The Lamaholot (and only the Lamaholot) refer to this overdyeing process, which is essential for all bridewealth textiles, as belapit (Ruth Barnes 1994, 20). The deep purple, which almost appears black, is called fange (Ruth Barnes 1994, 45).
However there are some exceptions to this rule. On both Ile Api and Savu, a dark indigo is achieved by an initial dyeing with morinda, followed by multiple immersions in indigo. In East Flores the bound ikat skeins known as kenuma for the kewaték mé’an bridewealth sarongs are always dyed with morinda first, and are then later partially overdyed with indigo (Graham 1994, 235).
Morindin and Morindone
The root bark of morinda trees that are over 3 years old contains from 0.25% to 0.5% of morindin, as well as many other anthraquinones and their glucosides such as:
- damnacanthal, an ether of lucidin (Hiramatsu et al 1993)
- chlorubin, rubiadin, and morindadiol (Elkins 1998)
- hydroxyanthroquinone, nordamnacanthal and lucidin (Ismail et al 1997)
- soranjidiol (Lacasse and Baumann 2004, 317)
Many of these are red, orange and yellow colorants (Jansen and Cardon 2005, 111; Ismail, Alias and Osman 2012, 298-301). However yellow lucidin, which also occurs in madder, has caused some concern since it is both mutagenic and carcinogenic (Schulz 2004, 292).
Both morindin and morindone were first discovered in 1849 by Thomas Anderson, who extracted a pale yellow crystalline substance from the roots of M. citrifolia and named it morindin. After distillation, morindin yielded a reddish yellow crystalline sublimate that Anderson termed morindone. The latter was completely insoluble in cold water, but readily dissolved in alcohol or ether.
Both Rochleder in 1852 and Stenhouse in 1864 mistakenly thought that morindone was alizarin (Thorpe and Greenall 1887; Thorpe and Smith 1888; Perkin and Hummel 1894; Oesterle and Tisza 1907). In 1925, Adams and Jacobson finally proved that morindone was actually 1:2:5-trihydroxy-6-methyl-anthraquinone:
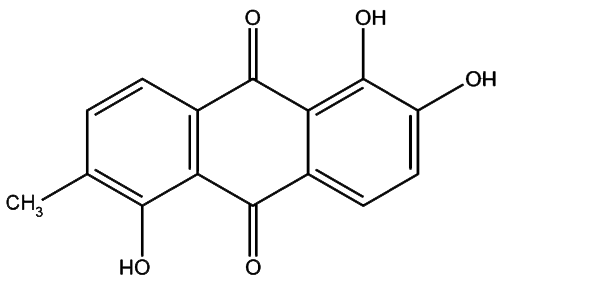
The molecular structure of morindone (above) and morindin (below)
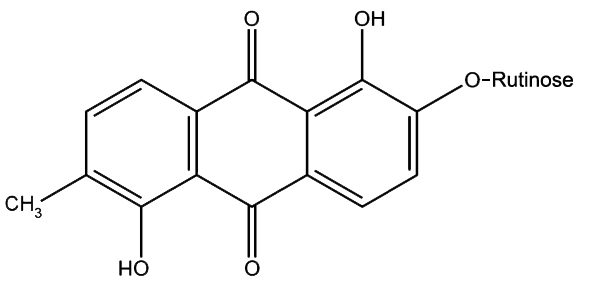
Morindin is a glycoside of morindone, composed of morindone and the disaccharide rutinose, the latter being frequently found in many naturally occurring flavonoids. Morindin is therefore more accurately termed a rutinoside of morindone.
Morindin is only partially soluble in cold water, but does dissolve in hot water. However in the latter case it precipitates on cooling as a gelatinous mass of β-morindin (Gmelin 1864, 190; Crookes 1874, 391). It does dissolve in weak alkalis, when it turns a deep orange-red.
Morindin is easily hydrolysed into morindone and glucose. This can be done:
- by chemical acid hydrolysis, for example using a weak mineral acid such as sulphuric acid, or
- by enzymatic alkaline hydrolysis, utilising a naturally occurring enzyme within the root bark to catalyse the hydrolysis reaction
The Anthraquinone Dyes
Both morindin and morindone are anthraquinones, a family of phenolic chemicals that include many other dyestuffs and pigments some natural such as alizarine, the active ingredient of madder, and others synthesised from anthracene extracted from coaltar.
All of the important natural red dyes are anthraquinones. Their main advantages are their brightness and relative fastness, especially light-fastness. Their disadvantage is that they are tinctorially weak.
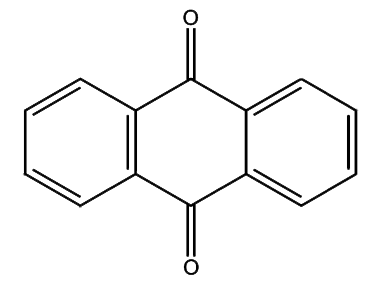
The molecular structure of anthraquinone
The most important natural anthraquinone dyes other than morinda include:
- madder root from plants belonging to the Rubiacceae or madder family, especially Rubia tinctorium. Madder is found in Europe, Turkey, the Middle East and Central Asia. The main constituent pigments are alizarin, purpurin, pseudopurpurin, morindone, xanthopurpurin, rubiadin and lucidin (Eastaugh, Walsh, Chaplin and Siddall 2008, 250).
- chay root from Indian madder (Oldenlandia umbellata) found in India and Burma. The main pigments are alizarin (1,2-dihydroxyanthraquinone) along with 1,2,3-trimethoxyanthraquinone, 1,3-dimethoxy-2-hydroxyanthraquinone, 1,2-dimethoxyanthraquinone, and 1-methoxy-2-hydroxyanthraquinone (Ramamoorthy et al 2009).
- lac from an insect and its larvae (Kerria chinensis and Kerria lacca lacca) which feed on certain trees, especially figs. Found in India, Southeast Asia and Sumatra. It is claimed that stick lac is the most widely used red dye in mainland Southeast Asia (Prangwatthanakun and Cheesman 1987, 45). The main lac pigments are laccaic acid, erythrolaccin, isoerythrolaccin and deoxyerythrolaccin (Eastaugh, Walsh, Chaplin and Siddall 2008, 220).
- kermes from the eggs of a parasitic insect (Kermes vermilio and Kermes ballotae) which lives on the scarlet oak tree. It is indigenous to Mediterranean Europe. The main pigment is kermesic acid, the aglycone of carminic acid (Hendry and Houghton 1996, 322).
- cochineal from a number of different scale-insects such as Porphyrophora polonica, found in Eastern Europe, or Dactylopius coccus found in Central and South America. The main pigment is carminic acid (Hendry and Houghton 1996, 322).
The basic chromophore of the anthraquinones is based on the resonance structure formed by the two carbonyl groups in conjugation with the delocalised carbon-carbon bonds in the two aromatic rings. Anthraquinone dyes are not only red, but can also be violet, blue and turquoise (Shuttleworth and Weaver 2013, 111).
Red anthraquinones absorb light in the blue visible region, with a λmax of around 430-450nm, due to excitation of the carbonyl groups (Ismail, Alias and Osman 2012, 296). The addition of an alkali such as dilute sodium hydroxide ionises the hydroxyl groups and leads to a bathochromic shift in the λmax absorption maxima. Hydroxyl groups in the 1- and 5-positions are hydrogen-bonded to the adjacent carbonyl group and so are more difficult to ionise than those in say the 2-position (Gordon and Gregory 2012, 197).
Anthraquinones also have characteristic absorptions in the ultraviolet at 265-280nm and 285-290nm. A study of morindone extracted from M. augustifolia found the strongest absorption to be in the 200-280nm ultraviolet range, with a secondary absorption band with its peak in the blue at 450nm (Aobchey, Sriyam, Praharnripoorab, Lhieochaiphant and Phutrakul 2002).
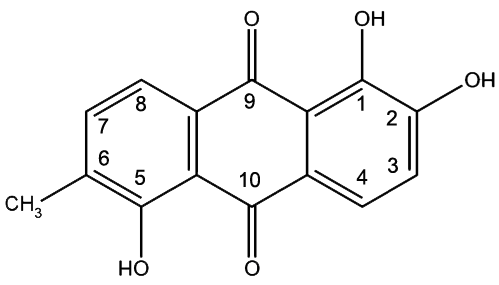
Morindone - 1:2:5-trihydroxy-6-methyl-anthraquinone
The hue of individual anthraquinone dyes is essentially controlled by the presence of electron donors, such as amino or hydroxyl groups in the 1-, 4-, 5-, and 8-positions of the anthraquinone skeleton (Shuttleworth and Weaver 2013, 111). Donors in the other substitution positions have less influence on hue, but can influence fastness or dyeing properties. In general those containing hydroxyl groups are more lightfast than those containing amino groups, at least on synthetic fibres.
Most anthraquinones also have an intense infrared absorption band in the 1600-1575 cm-1 region, which overlaps the carbonyl peak in highly chelated derivatives (Thompson 2012, 68).
The Chemistry of Morinda Dyeing
The biochemistry of the morinda dyeing process is not fully understood and has yet to be analysed scientifically.
Three of the earliest morinda studies were conducted in 1884 by Perkin and Hummel, using Indian M. umbellata; in 1907 by Barrowcliff and Tutin, using M. longiflora; and also in 1907 by Oesterle and Tisza using M. citrifolia. The former established that the active ingredient in the root was the glucoside morindin, along with five other anthraquinones. Morindin did not dye directly, but had to be first hydrolysed into the active pigment. This could be achieved by either boiling it with acids or alkalis, or by fermentation. The latter was considered by far the best route and required nothing more than adding the powdered root to water and allowing it to stand for about two hours. This was repeated twice more, extending the final steeping up to twenty hours. After the addition of sodium carbonate or chalk, the mixture was finally boiled and cleared with a solution of soap.
Perkin and Hummel believed that the washing helped remove certain acid substances that inhibited the dyeing process. They also discovered that the addition of different metal mordants resulted in the production of different colours. Using cotton calico treated with oil, an aluminium mordant produced a very bright orange-red or scarlet, chromium produced chocolate=brown and iron produced dull purple to black.
While not elucidating the chemistry of morinda dyeing Nobuko Kajitani, former conservator-in-charge at the Metropolitan Museum of Art in New York, has clarified the conditions required for different stages of the process as conducted in Indonesia (Kajitani 1980, 311 and 318). She suggests that the long pre-treatment process involving a cycle of oiling, sun-drying and moistening with dew not only allows the oil to penetrate the yarn but also oxidises it before it is eventually removed in the final alkaline wash. She also noted that while the pigment had to be extracted in an alkaline environment, the pH of the dyeing process depended on the fibre. Slightly acidic conditions were required to dye silk, but slightly alkaline conditions were required to dye cotton. Morinda dyed cotton red in the presence of an aluminium mordant, but brown in the presence of an iron mordant. Additional mordanting agents included fatty acids and tannin.
The need for alkaline conditions to dye cotton contrasts sharply with the curious claim of one team that ‘Indonesian traditional textile dyers seldom add an alkaline agent to the vat and, in practice, the morindin lake [morindone-aluminium complex] is mildly acidic’ (Cunningham, Maduarta, Howe, Ingram and Jansen 2011). In our experience, dyers almost always add alkali to the dye bath.
It has been suggested that the oiling pre-treatment is not part of the mordanting process, but is simply a scouring technique to remove the outer oils and waxes from the cotton fibres (Gavin 2013). This cannot be so – morinda is normally applied to yarns that have been long degreased and have often already been dyed with indigo.
It is more likely that the process of steeping the cotton yarns in oil and subsequently drying them chemically modifies the surface of the cotton fibres, making them more receptive to the morindone-aluminium dye complex. Cunningham’s team postulate that the fatty acids are oxidised and then form an elastic layer around the cotton fibre (Cunningham, Maduarta, Howe, Ingram and Jansen 2011).
The natural oils and fats used in the oiling process are complex cocktails of chemicals, chiefly composed of triacylglycerols, sometimes referred to as triglycerides. These are composed of three fatty acid esters linked together by a single glycerol molecule. In simple triacylglycerols the three fatty acids are the same, but most natural triacylglycerols are composed of two or three different fatty acids. The action of naturally occurring enzymes, called lipases, in the oil-water emulsion probably hydrolyses the ester bonds in the triacylglycerol thus releasing the fatty acids from their glycerol anchor.
A triacylglycerol composed of three different fatty acids
Fatty acids are carboxylic acids with a long aliphatic (non-cyclic) hydrocarbon chain. Most naturally occurring fatty acids have a chain with an even number of carbon atoms, ranging from 12 to 28. In saturated fatty acids the hydrocarbon chain is joined by single chemical bonds, but in unsaturated fatty acids it is joined by a mixture of single and double bonds.
The type and proportion of fatty acids in the vegetable oil varies depending on its nature, the growing conditions of the host plant, the time of harvesting, and so on. For example, one analysis of Malaysian candlenut oil found that it contained: 54% stearic acid, 20% linoleic acid, 16% oleic acid and 6% palmitic acid (Norulaini, Budi, Omar, Zaidul and Omar 2004). An earlier analysis of Ceylon candlenut oil found that it contained 43% linoleic acid, 29% oleic acid, 24% linolenic acid, and 5% saturated acids (Child 1941).
Stearic acid is saturated, and so has no double bonds. Oleic, linoleic and linolenic acids are unsaturated, possessing one, two and three double bonds respectively.

Stearic acid C17H35-COOH above, and oleic acid C9H18=C8H15-COOH below
Natural oils from seeds and nuts are easily degraded by autoxidization in the presence of air, a chain reaction accelerated by light and/or heat. Oxidation primarily affects unsaturated fatty acids, free radicals cleaving their double bonds to produce volatile aldehydes and ketones.
Because this process is so important to the food industry, it has been analysed in detail. The reaction of oxygen with unsaturated fatty acids, written schematically as RH, is a typical chain reaction, involving initiation, propagation and termination. Initiation is caused by light, heat or the presence of trace metals and involves the removal of a hydrogen radical (proton), H*, from one of the –CH methyl groups on each side of the double bond (the most weakly bound hydrogen atoms). The resulting fatty acid radicals R* react with oxygen O2 to form peroxy radicals ROO*. In the propagation process the ROO* react with more fatty acids RH to form hydroperoxides, ROOH, the primary products of autoxidation (Frankel 1984).
The decomposition of these fatty acid hydroperoxides is extremely complex, producing a multitude of breakdown products such as aldehydes, ketones, alcohols, hydrocarbons, esters, furans and lactones (Frankel 1984).
Fatty acids can also be photo-oxidised by a different mechanism, requiring the presence of a sensitizer such as chlorophyll. This is an extremely fast process that depends on the formation of highly reactive singlet oxygen (Frankel 1984).
Because it has three double bonds, linolenic acid oxidises more rapidly than linoleic acid, which only has two. Likewise linoleic acid oxidises more rapidly than oleic acid, which only has one (Zeleny and Coleman 1937, 32). The relative rate of autoxidation of oleic, linoleic and linolenic acids has been reported to be of the order 1:12:25 on the basis of peroxide formation and 1:50:100 on the basis of oxygen uptake (Frankel 1985). Oils that contain a higher proportion of unsaturated fatty acids are likely to be more effective than those that do not.
The addition of the alkali lye brings about a limited cold saponification, a process that is catalysed by the presence of certain phenols. The fatty acids react with the mineral alkali to form salts, while fatty acid esters are decomposed into the fatty acid salt and an alcohol. Fatty acid salts are anionic surfactants, which are soluble in water. However it is not clear how the fatty acid salt binds to the cellulose surface. Possibilities include hydrogen bonding or Van der Waals forces.
Sodium stearate, showing the hydrophilic negatively charged carboxyl group
(ChemWiki, University of California, Davis)
Cellulose cotton fibres contain polar hydroxyl groups that develop negative charge in neutral or alkaline solutions. In fact the presence of alkali makes the cellulose even more anionic. Unlike cationic surfactants, anionic surfactants such as fatty acid salts are not absorbed well onto negatively charged cellulose. They can only absorb onto it with their negatively charged hydrophilic carboxylic group oriented away from the similarly charged cellulose polymer (Rosen and Kunjappu 2012, 409). It is possible that the carboxyl groups on the adsorbed fatty acid salts act as binding sites for the aluminium-morindone dye complex.
We must emphasise that the purpose of this discourse is not to explain how the process of oiling modifies the surface chemistry of cellulose, but to highlight the complexity of the chemistry involved. The key chemical changes that assist the morinda dye complex to bind to the cellulose fibres still remain a complete mystery, although we can see some of the mechanisms of how they might be brought about.
The final metal mordanting process is far easier to understand, at least in principle. In the case of the two yellow flavonols, morin and quercetin, it has been demonstrated that the addition of increasing concentrations of aluminium mordant not only brings about a bathochromic shift in light absorption but also leads to increasing absorption of light at the absorption maxima, λmax (Septhum, Rattanaphani and Rattanaphani 2007). This is due to the creation of an increasing concentration of flavonol-aluminium complexes in the dye bath. The mordant not only creates complexes that have a greater bonding strength with the substrate than the dye alone, but also intensifies the colour of the dye itself.
It seems likely that a similar process takes place with anthraquinones such as morindone. Although there have been many studies on the chelation of anthraquinones with transition metals, we are unaware of any study specifically focussed on morindone and aluminium. However it is easy to postulate how the adjacent carbonyl and hydroxyl groups might chelate with a metal ion:
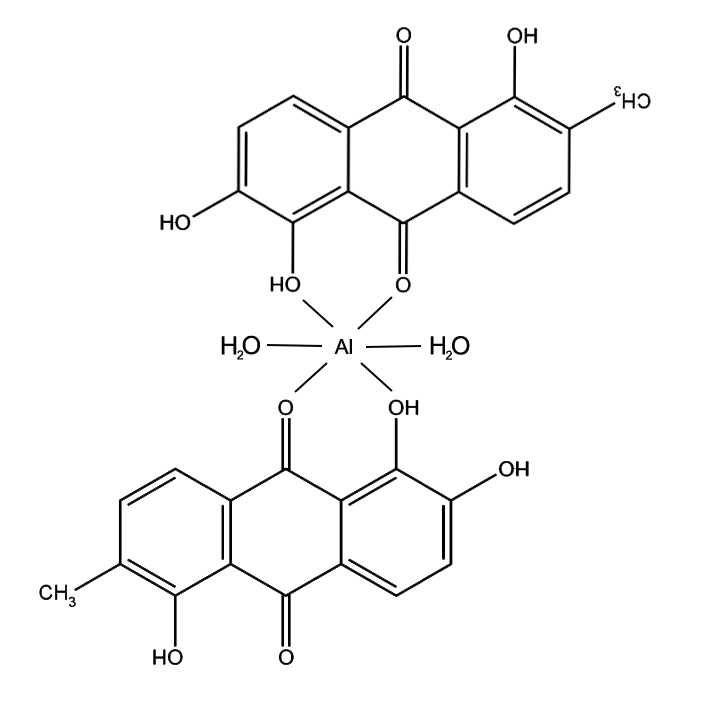
Hypothetical complex between two morindone molecules and an Al+++ ion
Morinda Brown or Morinda Red?
Depending on the details, length and complexity of the dyeing process, morinda can produce a range of shades from pink to orange, red and brown. When we look at different regions across Indonesia, the locally dyed morinda tends to have a characteristic shade. For example, the old ikats of Toraja, Sulawesi, tend to include deep reddish-brown colours - although the shade tends to be a more orange-brown in modern renditions. Likewise in Tenganan Pegringsingan, Bali, where dyers previously invested an enormous length of time in treating their yarns with morinda, they produced a deep orange-brown tone.

This porisitutu shroud from Rongkong, south Sulawesi,
has deep reddish-brown tones of morinda
(Image courtesy of the Asia-Pacific collection at Yale University Art Gallery)

This geringsing saput with the cicempaka magnolia flower pattern from Tenganan Pegringsingan, Bali, has dark orange-brown tones
However the situation is not entirely black and white. Although individual regions tend to have their own morinda tones, we can sometimes still see differences between cloths produced at the same time within the same village, some morinda appearing red and some brown. This is particularily noticeable today among the Amarasi in West Timor. These differences must relate to the specific recipes followed and the materials selected by individual dyers.
In the Lesser Sunda Islands we find that morinda tends to appear a rust brown or chestnut brown in regions such as Ende, Sikka, East Flores, Adonara, Solor, Lembata and Alor, all of which are located along the chain of northern volcanic islands. However morinda tends to appear red in the more southern limestone islands such as Savu and Sumba, as well as West Timor and Semaru.
The reasons are not entirely clear. The achievement of a deep morinda red depends crucially on the rigorous pre-treatment of the cotton yarns by repeatedly steeping them in a mix of oil and alkali, thoroughly drying them in between. It seems that in locations such as Lembata, this pre-oiling process may not be as intensive as it is in places such as Savu or East Sumba.
Another factor might be the quality of the local water. On the volcanic islands dyers tend to use water from mountain streams, whereas on the limestone islands of Savu and Sumba they also use water from streams, but often from wells and boreholes during the dry season. Water on the volcanic islands is likely to be acidic and rich in salts containing sodium, potassium, chloride, sulphate, and silicate ions. Water on the limestone islands is likely to alkaline and to be much harder, with salts containing calcium, magnesium, carbonate, bicarbonate, and to a lesser extent sulphate ions. From the investigations into the very similar chemistry of Turkey Red dyeing we know that the active pigment alizarin forms quite different metal complexes with small ions like sodium than it does with larger ions like calcium.
The colour of morinda can also be altered if it is applied to ikatted yarns that have initially been dyed with indigo - even though the some of the morinda-dyed sections were bound during the indigo dyeing stage. This is because some of the indigo from the sections overdyed with morinda is washed into the morinda dye bath and darkens the morinda that adheres to the undyed sections of yarn, producing a deeper reddish brown (Tamu Rambu Hamu Eti and Hambuwali 2016, personal communication).
Regional colour difference may also depend on whether dyers finish the morinda process with a final stage involving the addition of other dyewoods and in some cases tannin. Thus at Ndona they still add jackfruit to the final dye bath to deepen the reddish-brown colour, while the Endenese gave their morinda yarns a final immersion in a hot dye bath containing morinda, sappan and Symplocos in order to achieve a browner shade (Cunningham, Maduarta, et al 2011). In Sikka some dyers added the bark of rawamatan, while at Doka today they add mango and jackfruit bark. On Ile Api some dyers treat their yarns in a bath of boiled tamarind. Finally in West Timor, some Amarasi dyers completed the morinda dyeing process with an after treatment of sappanwood and tree lichen (Bühler 1948, 2499).

Brown colours produced by morinda. From top left to bottom right: Lio, Sikka, East Flores and Ile Api, Lembata

Red colours produced by morinda. From top left to bottom right: Amarasi, Helong, Savu and East Sumba
These differences not only highlight the amazing character and versatility of natural dyes, but also add to the joy of studying and collecting textiles from this incredible region.
Photo-Oxidation
Unfortunately morinda has only been included in a few comparative studies of dye light-fastness. From the limited information available, morinda appears to be moderately lightfast and wash-fast. It is certainly more stable to light than all of the natural yellow dyes, and probably rates alongside indigo - while remaining inferior to the majority of modern synthetic red dyes.
The stability of the anthraquinone chromophore depends on the position and nature of the substitution groups in the anthraquinone skeleton. Those like morindone, which have an OH or NH group in the 1-, 4-, 5- or 8- positions adjacent to one of the carbonyl groups, are more resistant to photo-oxidation because of hydrogen bonding between the carbonyl oxygen atom and the adjacent OH or NH group (Davis, Douglas, Evans and Burrows 2014, 153). However those that are 2-substituted have very low light-fastness and make unsuitable dyes (Allen and Edge 1992, 186).
The light-fastness of anthraquinones primarily depends on how slowly they are photo-oxidized in the presence of air (oxygen). It has been proposed that photo-excited π-bonds transfer energy to dissociate molecular oxygen, which then either hydrolyses or oxidises the anthraquinone skeleton (Hihara, Okada and Morita 2002, 174). However even in an anaerobic environment, visible and UV light can also cause photo-reduction. Photo-reduction occurs due to photo-excitation of the carbonyl groups, which then harvest hydrogen from the cellulose substrate (Hihara, Okada and Morita 2002, 174). It seems that red and yellow anthraquinone dyes are far more photosensitive than blue, green and olive anthraquinone dyes because they requires more energetic light in the blue and UV region to excite their π-bonds.
One of the earliest investigations into the light-fastness of natural dyes was conducted between 1890 and 1899 by a committee appointed by the British Association for the Advancement of Science. The dyes were measured against five different standards ranging from class I to class V. Class V dyes were the most light-fast and showed little fading after one year of exposure to daylight in a village close to Leeds. Using a substrate of wool, the six red anthraquinone dyes morinda, madder, chayroot, cochineal, kermes and lac were placed into classes III, IV and V. All of the yellow dyes were placed into classes I and II, while indigo was rated class V (Padfield and Landi 1966). It is likely that these dyes would be less light=fast on cotton.
A more recent study found that cotton dyed with morinda was more stable to light than that dyed with madder, indigo, or turmeric. The measured colour change per thousand lux hours was around 6 for morinda, 5 for sappanwood, 13 for madder, 15 for indigo, 17 for ferric mud and 28 for turmeric (Ford 1992). The light=fastness of morinda extracted from M. augustifolia dyed on cotton using an aluminium mordant was rated 4 on a scale of 1 to 6 (1 = very poor, 6 = very good). Wash-fastness was rated 5 (Bhuyan, Saikia and Saikia 2001).
The latest study rated the light-fastness of aluminium-mordanted morinda on cotton as 3 to 4 against the Indian Standard 2454, which is calibrated against eight standard blue dyes (1 = poor, 8 = excellent) (Patel 2011). Wash-fastness was rated 4.
Turkey Red
We can gain some insights into the chemistry of morinda dyeing by looking at the very similar ‘Turkey Red’ process, which was used for dyeing cotton using madder root rather than morinda root. Both processes involve two very similar red anthraquinone dyes - alizarin from madder and morindone from morinda. Turkey Red produced brilliant shades of red that were highly durable. They were not only very light- and wash-fast but also resisted traditional bleaching with agents such as chlorine. Edward Bancroft compared the vivacity and beauty of Turkey Red to that of the finest cochineal scarlet (Bancroft 1814, vol. 2, 172). European dye houses strove to import and replicate this complex dye process. Having done so, they encountered many problems - especially when they tried to simplify it by eliminating certain stages or reducing the number of repetitive steps.
The dyeing of Turkey Red was a highly intricate process involving dozens of stages and taking over three months to complete. It involved a lengthy initial alkaline oiling stage, followed by pre-mordanting with aluminium and tannin and then a final dyeing sequence with madder root in weak alkaline solution in the presence of more aluminium mordant.
Jim Liles has summarised the traditional process for dyeing cotton yarn with morinda as described in early dye books published prior to 1870 (Liles 1990, 113-114):
- scour well for several hours in soda ash or pearl ash (potassium carbonate)
- rinse and dry
- soak in an emulsion of vegetable oil, or fat and soda ash or pearl ash. Sometimes dung was added to the first oiling
- wring out and hang wet for three days, preferably drying in bright sunlight during the day and dampening by dew overnight
- repeat the oiling six or seven times
- rinse repeatedly with soda ash or pearl ash to remove all of the free oil and finally rinse and dry
- soak in weak tannin solution, dry and then mordant once or twice with alum and alkali. Dry overnight. Some dyers combined the tannin and alum into one solution
- some dye workshops repeated the whole process again at this point, starting with the oiling
- wash in a fixing solution of weakly alkaline dung, which contains calcium and sodium phosphates
- without drying, dye with madder, chalk or calcium acetate, and a small amount of tannin. Some dyers added a small amount of ox blood
- some dyers repeated the madder dyeing stage a second time
- boil for several hours in a soap solution containing soda ash or pearl ash. Repeat this stage a second time
- rinse and dry in strong sunlight

Samples of calico dyed with Turkey Red from the Alexandria Print Works, Vale of Leven, Scotland, 1886-1888 (University of Glasgow 2013)
The History of Turkey Red
This missing section will be added shortly.

The Delaunay Turkey Red Dyeworks at Blackley, near Manchester, painted by K. Littlejohn
(Image courtesy of Carole Paterson)
The Chemistry of Turkey Red Dyeing
Early European and American dyers were baffled by the complexity of the Turkey Red process. In 1794 the celebrated American authority on natural dyes, Dr. Edward Bancroft, noted in his Philosophy of Permanent Colors that Turkey Red dyeing was an ‘unaccountable process that chemical analysis does not elucidate’.
It was well known that cotton was difficult to dye, whereas animal fibres like wool and silk were not. Dyers therefore assumed that cotton needed to be endowed with the same properties as animal fibres, before it could be properly dyed. This pre-dyeing process was given the name ‘animalisation’. As early as 1763 the Abbé Guillaume Mazèas of Brittany proposed that the process for dyeing red used by the artisans of Malabar and Coromandel depended firstly on this process of animalisation, and secondly on the strong affinity of madder-like dyes to certain types of animal matter (Monthly Review May 1791, 15). A little later, in 1786, Thomas Henry put forward a similar theory for the dyeing of Turkey Red (Monthly Review May 1791, 11-15). He believed that the most essential part of the process was the impregnation of the cotton fibre with animal matter so that it acquired the same properties of animal fibres.
In 1807 Jean-Antoine Chaptal emphasised that the mordant consisted of three elements – oil, a principle astringent such as gallnut, and aluminium. He concluded that ‘this mordant, undoubtedly, is the most complex of any which is known in dyeing; and it presents to chemists a sort of combination eminently deserving of their utmost attention’ (Bancroft 1814, 201). In 1815 Thomas Cooper emphasised that the purpose of mixing the alkali and oil was to produce an imperfect soap, in other words a soap that is not as stable as a proper soap made with a stronger alkali such as lime (Cooper 1815, 297).
In 1816 John Thompson of Glasgow rejected the idea of animalisation, noting that the sheep’s dung added to the initial oiling mixture contained very little animal matter. Recognising that unlike cotton, the fibres of silk and wool had a natural varnish, he proposed that the oil absorbed oxygen to form a layer of varnish on the cotton surface to which the dye combined (Thompson 1816, 463-465). This explained why the permanency of the madder was proportional to the number of immersions in the oil-alkali emulsion – each immersion increased the amount of oil ‘imbibed’, effectively laying on successive coats, one above the other. The purpose of the dung was simply to keep the fibres moist so that they could adsorb the oil. Thompson believed that the addition of blood was superfluous.
In 1873 the Société Industrielle de Mulhouse, the great Alsatian textile-dyeing centre, offered a prize for an essay on the theory of the manufacture of Turkey red (Chemical News 17.10.1873, 205). In 1876 Theodor Chateau proposed the theory that the essential ingredient provided by the oil was a mixture of fatty acids, out of which oleic acid was predominant. The addition of alumina formed the aluminium fatty acid salt, which then combined with the vegetable ‘albumen’ in the oil and the animal ‘albumen’ from the dung, egg yolk, blood and gall. This triple compound united with the dye but only weakly bound with the surface of the cotton fibre (Chemical News 03.11.1876, 196). In 1902 Driessen suggested that an oxidised oil would be impossible to apply to the fibre because it would be insoluble. He proposed that the oil was not modified by the alkali and combined with the cotton fibre so intimately that a range of solvents could not remove it. Its effect was to ‘animalise’ the fibre so that the alumina would then bind to its surface (Driesen 1902, 178-179).
In 1868 two German chemists at BASF, Graebe and Liebermann, successfully synthesised alizarin from the coal tar by-product anthracene (Reinhardt and Travis 2013, 151). By 1871 alizarin was already being produced by Hoechst and Gebrüder Gessert in Germany, and by Perkin and Sons in London. By 1875 the commercial product had almost totally supplanted natural European madder, mainly grown in the Languedoc region of France (Aftalion 2001, 45). Most dye specialists quickly lost interest in trying to understand the chemistry of the Turkey Red process.
However in 1940 two Swiss dye chemists, Hans Eduard Fierz-David and Max Rutishauser, attempted to identify the structure of the Turkey Red alizarin ‘lake’, the term used by dyers to refer to the anthraquinone and metal mordant dye complex. After isolating the complex they concluded that it contained alizarin, aluminium and calcium in the proportions 4:2:3 (Taha 2014, 21-2).
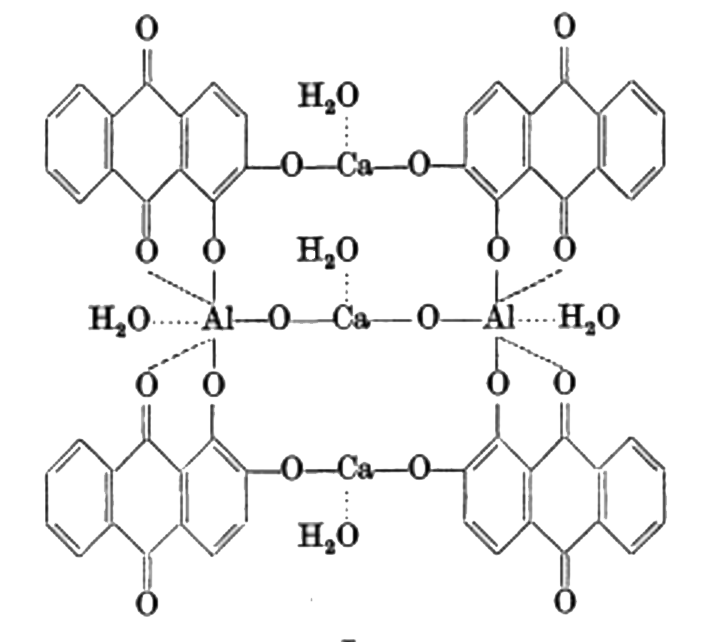
The structure proposed by Fierz-David and Rutishauser (Schweiser 2013, 304)
Kiel and Heertjes challenged this conclusion in 1963. Using infrared spectroscopy they analysed the aluminium-alizarin complex in aqueous solution and found that it was formed from one atom of aluminium, one atom of calcium and two molecules of alizarin (Kiel and Heertjes 1963; Zollinger 2003, 255; Allen 2013, 144-145). They proposed it was the calcium salt of a mononuclear aluminium-alizarin complex containing one aluminium atom chealated in the β-ketol position to two alizarin molecules:
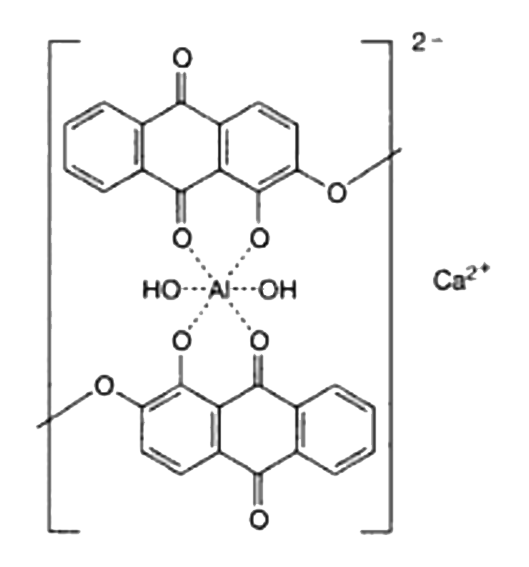
In 1994 Wunderlich and Bergerhoff conducted an X-ray crystallographic study of an aluminium-alizarin complex crystallised from dimethylformamide. They concluded that the complex was polynuclear bonded. Two aluminium cations and two calcium cations were chelated to four alizarin molecules, with the added twist that the two central aluminium ions were linked by hydroxyl bridges.
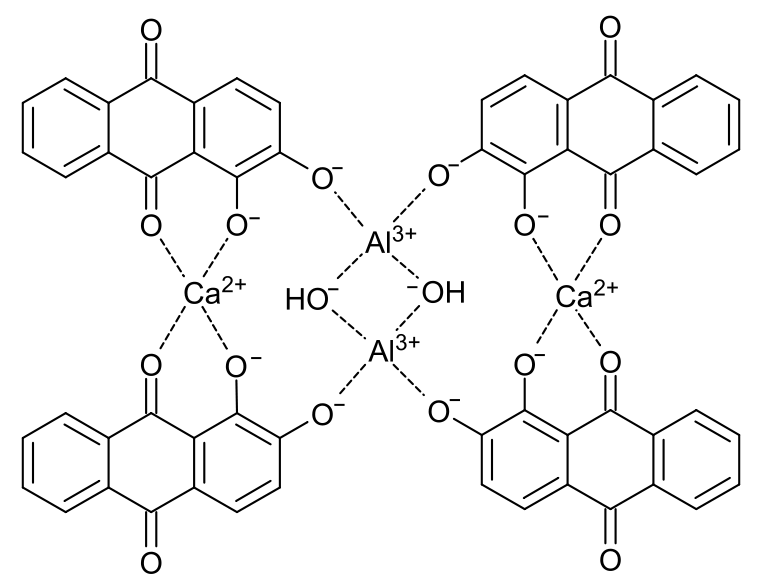
Aluminium-alizarin complex according to Wunderlich and Bergerhoff, 1994
In 1996 Patrick Soubayrol, Gilbert Dana and Pascal Man conducted a novel study of aluminium-alizarin complexes in the solid state, using nuclear magnetic resonance (NMR) - a useful means of analysing the coordination of aluminium in metal complexes. They synthesised the complexes using four different alkalis – sodium hydroxide, potassium hydroxide, calcium hydroxide and barium hydroxide. Two distinct families of binuclear aluminium-alizarin complexes were identified, both containing hexa-coordinated aluminium: those with closed structures and those with open structures.
Closed structures are formed with smaller sodium or calcium ions, the alizarin molecules folded into a tetrahydrate structure, their eight carbonyl oxygen atoms bound to the four water molecules and with two of the four sodium ions or both the calcium cations confined inside, sandwiched between two benzene rings in opposing alizarin molecules.
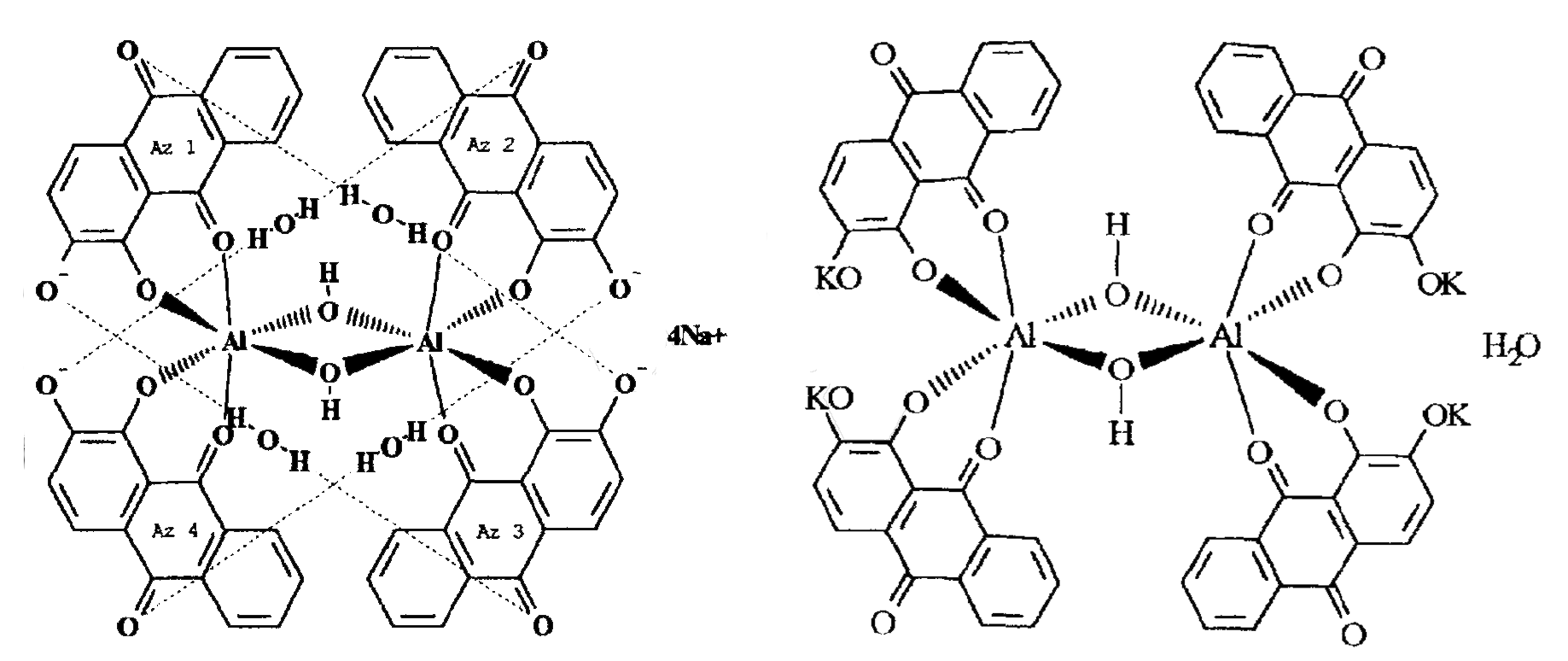
Left, one of the closed tetrahydrate complexes, right, one of the open complexes, in this case a monohydrate (Soubayrol et al 1996)
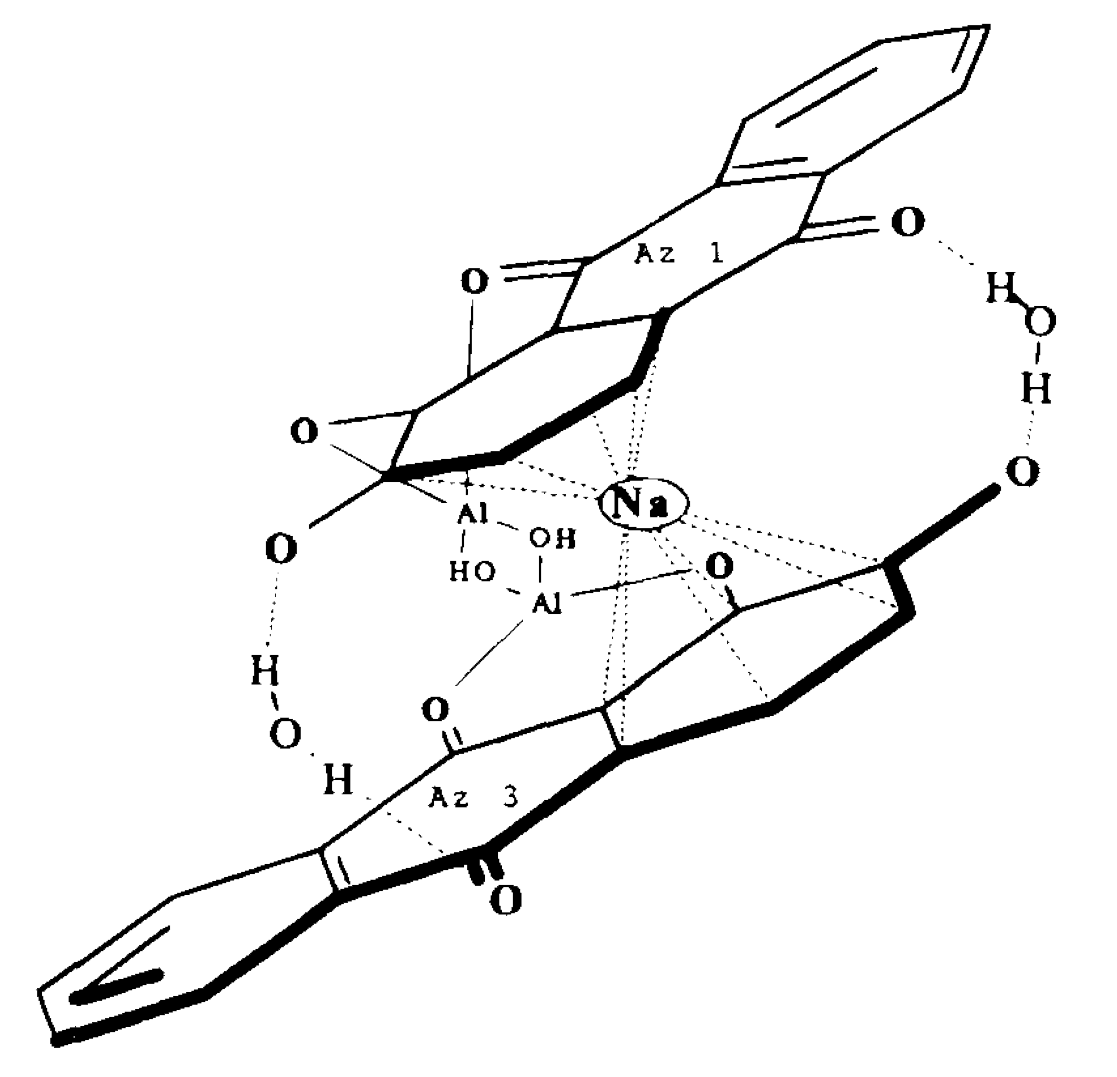
Perspective view of one half of the tetrahydrate complex, with one sodium cation sandwiched between the benzene rings on two opposing alizarin molecules (Soubayrol et al 1996)
Open structures are formed with bulkier cations such as potassium or barium, which are too large to fit inside a closed alizarin structure. Consequently these structures remain open, and the four cross-linking water molecules are no longer required.
Soubayrol et al suggest that the closed structures may explain how such aluminium-alizarin complexes can bind with a cellulose fibre. It is possible for the two water molecules in the alizarin sandwich to be replaced by a cellobiose unit of a cellulose polymer within the cotton fibre. However this hypothesis is concerning, because it does not explain how the important oiling stage contributes to the process.
Stereoscopic view of a closed alizarin tetrahydrate complex binding with two cellubiose units, one above and one below (Soubayrol et al 1996)
Clearly more work is required, but at this stage it seems that there may be a range of different aluminium-alizarin complexes, depending on the conditions of the dye bath, especially the nature of the alkali used.
The primary chelating sites appear to be the 1-hydroxyl and adjacent 9-carbonyl groups, and not the adjacent 1- and 2-hydroxyl groups. However the 2-hydroxyl group does play a secondary role in the polynuclear structure identified by Wunderlich and Bergerhoff and the closed structures identified by Soubayrol et al.
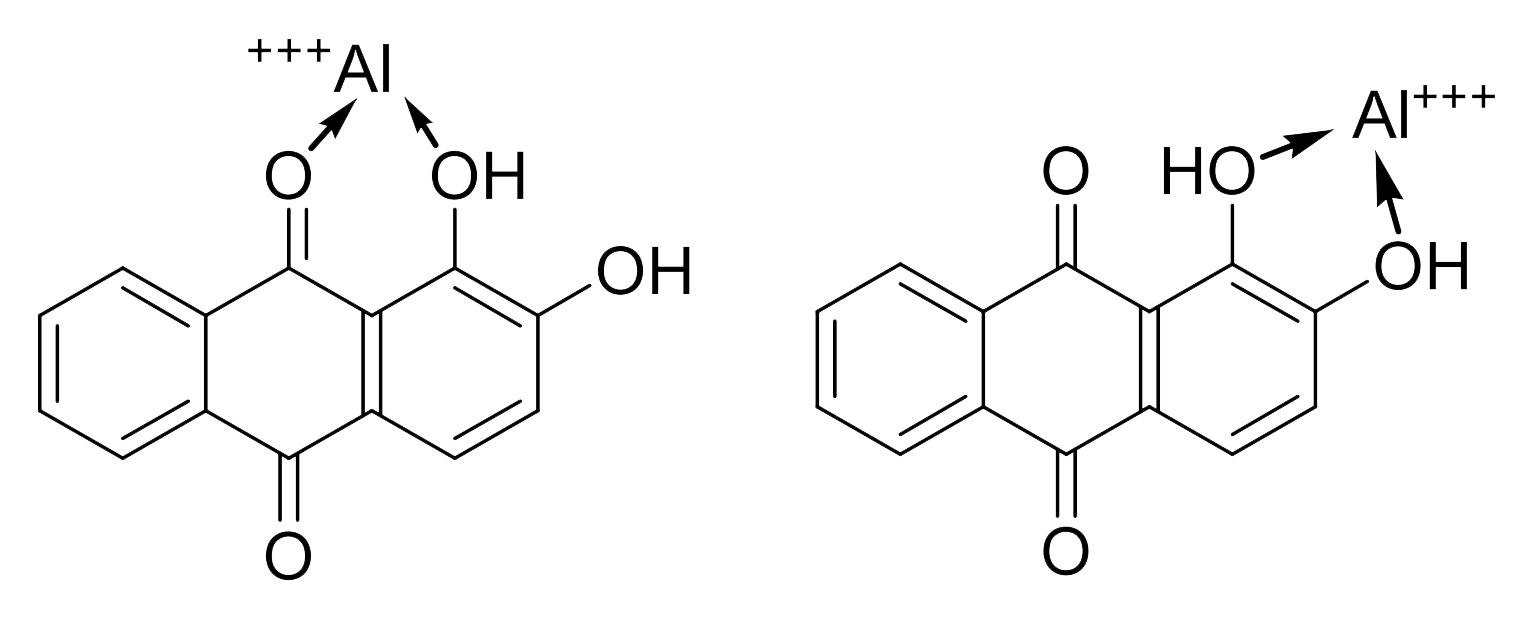
The main aluminium chelating sites are the adjacent hydroxyl and carbonyl groups (keto-phenolate), left, not the adjacent hydroxyl groups (di-phenolate), right.
It was subsequently found that when aluminium concentrations are relatively low in relation to the alizarin concentration, the aluminium-alizarin complexes could polymerise into linear chains. These chains can then cross-link as a result of π-π interactions between the aromatic rings to form a gel or lacquer (Sanyova 2001).
These laboratory studies have been confined to examining the reaction between just alizarin and aluminium. However the real dye lake formed by the use of natural materials is a far more complex chemical cocktail of pigments. A Belgian study has investigated a nineteenth century madder paint from Sweden, using hydrogen fluoride to extract the aluminium from the various dye complexes in order to release and identify its constituent colourants (Sanyova and Reisse 2006). The main component was ruberytheric acid, the glycoside of alizarin, accompanied by the glycosides of rubiadin, lucidin and quercetin - all anthraquinone pigments. The sample also contained alizarin and purpurin, along with smaller amounts of pseudopurpurin and munjistin.
In 2014 the University of Glasgow Department of Organic Chemistry initiated a joint project with the Textile Conservation Centre entitled ‘Resurrecting Turkey red: Adapting a historic process for modern re-creation and analysis’. As part of the project, Julie Wertz has been reexamining the chemistry of Turkey Red. She realised that a major stumbling block had been the failure to understand that Turkey Red is not a single chemical entity that can be extracted from the fibre and analysed. Rather it is built up on the surface of the fibre layer by layer by a sequence of treatments. The structure of the dye needs to be analysed on the surface of the fibre.
Wertz has started analysing samples of Scottish nineteenth-century Turkey Red dyed textiles using diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS). This non-destructive technique requires no sample preparation and simply measures the absorbance spectrum of radiation scattered from the textile surface. It is particularly suited to analysing heterogeneous powders, polymers and fibres. The initial results show that oil is a key component of the Turkey Red pigment, appearing as an absorbance peak in the 1800-1600cm-1 region. However the function that the oil plays in the dye binding process is still yet to be elucidated.
Progress is slowly being made to our understanding of the Turkey Red process, although there is a great deal more still to discover about this intriguing dyeing technique.
Bibliography
Böhmer, Harold, 2002. Koekboya: Natural Dyes and Textiles, REMHÖB-Verlag, Ganderkesee.
Bühler, Alfred, 1948. Primitive Dyeing Methods, Ciba Review, vol. 68, pp. 2485-2500.
Clark, Matthew, 2011. Handbook of Textile and Industrial Dyeing: Principles, Processes and Types of Dyes, Cambridge.
Chaptal, Jean-Antoine, 1807. L’art de la teinture du coton en rouge, Deterville.
Cunningham, Anthony B.; Maduarta, I. Made; Howe, Jean; Ingram, W.; and Jansen, Steven, 2011. Hanging by a Thread: Natural Metallic Mordant Processes in Traditional Indonesian Textiles, Economic Botany, vol. XX, issue X, pp. 1–19.
Cunningham, A.B.; Ingram, W.; Kadati, W. Daos; Howe, J.; Sujatmoko, S.; Refli, R.; Liem, J. V.; Tari, A.; Maruk, T.; Robianto, N.; Sinlae, A.; Ndun, Y.; Made Maduarta, I.; Sulistyohardi, D.; and Koeslutat, E., 2011. Hidden economies, future options: trade in non-timber forest products in eastern Indonesia, Australian Centre for International Agricultural Research (ACIAR), Canberra.
Cunningham, Anthony B.; Kadati, Willy Daos; Ximenes, Jose; Howe, Jean; Maduarta, I. Made; and Ingram, William 2014. Plants as the Pivot: The Ethnobotany of Timorese textiles, in Textiles of Timor, Hamilton, Roy W., and Barrkman, Joanna, (eds), Fowler Museum, LA.
Driessen, Felix, 1902. Étude sur le Rouge Turc, Ancien Procédé, Bulletin de la Sociéte Industrielle de Mulhouse, vol. LXXII, pp. 163-180, Mulhouse.
Goodman, Sara; Garcia, Michel; and Ingram, William, 2013. The Plant Mordant Project: Natural Dyes 100% from Plants, Bebali Foundation, Ubud, Bali.
Hamilton, Roy W., 1994. 'Textile Technology', in Gift of the Cotton Maiden: textiles of Flores and the Solor Islands, Hamilton, Roy W., (ed.), pp. 58–77, Los Angeles.
Herawati, Tuti, and Adalina, Yelin, 2011. Local Knowledge of East Sumba People in Morinda Utilization as Natural Dye, Proceedings the 2nd International Symposium of Indonesian Wood Research Society, Bali, Indonesian Wood Research Society.
Heringa, Rens, 1989. Dye Process and Life Sequence: the Coloring of Textiles in an East Javanese Village, in To Speak with Cloth: Studies in Indonesian Textiles, University of California, LA.
Heyne, K., 1917. De Nuttige Planten van Nederlandsch-Indië, vol. 4, pp. 207-214, Batavia.
Hofmann, Regina, 1997. The Bühler Collection of Indonesian Dyeplants, in Dyes in History and Archaeology, vol. 15, pp. 3–26.
Hofmann-de Keijzer, Regina; and van Bommel, Maarten R., 2005. TLC and HPLC Analysis on Red and Violet Cotton Yarns of Indonesian Textiles, in Dyes in history and archaeology 20, London.
Hunter, William, 1799. On the Plant Morinda and Its Uses, in Asiatic researches or transactions of the Society instituted in Bengal, for inquiring into the history and antiquities, the arts, sciences, and literature, of Asia, vol. 4,
Ingram, William, 2014. The Plant Mordant Project: Bringing Indonesia’s Traditional Plant-sourced Dye Mordant to the World, in Resist Dye on the Silk Road: Shibori, Clamp Resist and Ikat, pp. 184-190, Hangzhou.
Janssen, P. C M., and Cardon, D., 2005. Plant Resources of Tropical Africa, vol. 3, Dyes and tannins, PROTA Foundation, Wageningen.
Kajitani, Nobuko, 1980. 'Traditional Dyes in Indonesia', in Indonesian Textiles: Irene Emery Roundtable on Museum Textiles 1979 Proceedings, Textile Museum, Washington, D.C.
Liles, J. N., 1990. The Art and Craft of Natural Dyeing: Traditional Recipes for Modern Use, University of Tennessee Press, Knoxville.
Nooteboom, H. P., 1975. Revision of the Symplocaceae of the Old World, New Caledonia excepted. Leiden University Press, Leiden, Netherlands.
Nooteboom, H.P., 1991. Symplocos Jacq., in Plant Resources of South-East Asia No. 3: Dye and tannin-producing plants, Lemmens, R.H.M.J. and Wulijarni-Soetjipto, N. (eds), pp. 115-118, Pudoc, Wageningen, The Netherlands.
Nooteboom, H. P., 2013. Symplocaceae, in The Families and Genera of Flowering Plants, vol. VI, Kubitzki (ed.), pp. 443-449, Springer.
Perkin, A. G. and Hummel, J. J., 1894. Colouring and other principles contained in mang-koudu, Transactions of the Chemical Society, vol. 65, p. 851.
Quattrocchi, Umberto, 2012. CRC World Dictionary of Medicinal and Poisonous Plants, CRC Press.
Razafimandimbison, Sylvain G.; McDowell, Timothy D.; Halford, David A.; and Bremer, Birgitta, 2010. Origin of the pantropical and nutriceutical Morinda citrifolia L. (Rubiaceae): comments on its distribution range and circumscription, Journal of Biogeography, vol. 37, issue 3, pp. 520-529.
Rumpf, Georg Eberhard, 1755. Herbarii Amboinensis, vol. 3, Amsterdam.
Saleha, Abu; Kamath, L. Manju; and Peter, P. I., 2007. Indian Noni (Morinda citrifolia L.): A Unique Fruit Crop with High Medicinal Value, in Underutilized and Underexploited Horticultural Crops, pp. 301-312, New Delhi.
Schaefer, G., 1941. The history of Turkey red dyeing, Ciba Review, no. 39, pp. 1407-16.
Septhum, Chutima; Rattanaphani, Vichitr; and Rattanaphani, Saowanee, 2007. UV-Vis Spectroscopic Studies of Natural Dyes with Alum as a Mordant, School of Chemistry, Institute of Science, Suranaree University of Technology, Thailand.
Subagiyo, Puji Yosep, 2007. Indonesian Natural Dyeing Recipes, Jakarta.
Verheijen, J. A. J., 1990. Dictionary of plant names in the Lesser Sunda Islands, Australian National University, Canberra.
Wang, Yuguo; Fritsch, Peter W.; Shi, Suhua; Almeda, Frank; Cruz, Boni C.; and Kelly, Lawrence M., 2004. Phylogeny and infrageneric classification of Symplocos (Symplocaceae) inferred from DNA sequence data, American Journal of Botany, vol. 91, no. 11, pp. 1901-1914.
Wertz, Julie, 2015. Cut from the same cloth? Unravelling the secret of Turkey red
Publication
This webpage was first published on 24th January 2016.